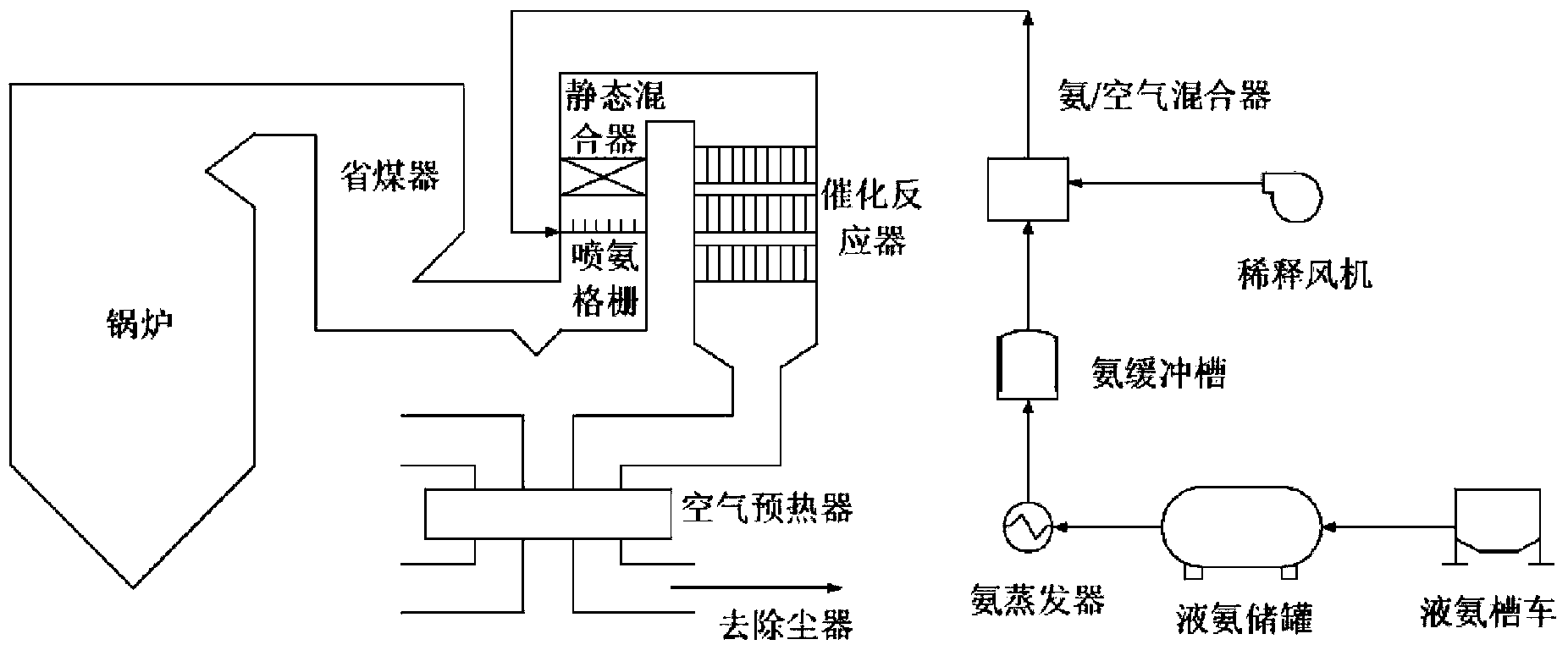
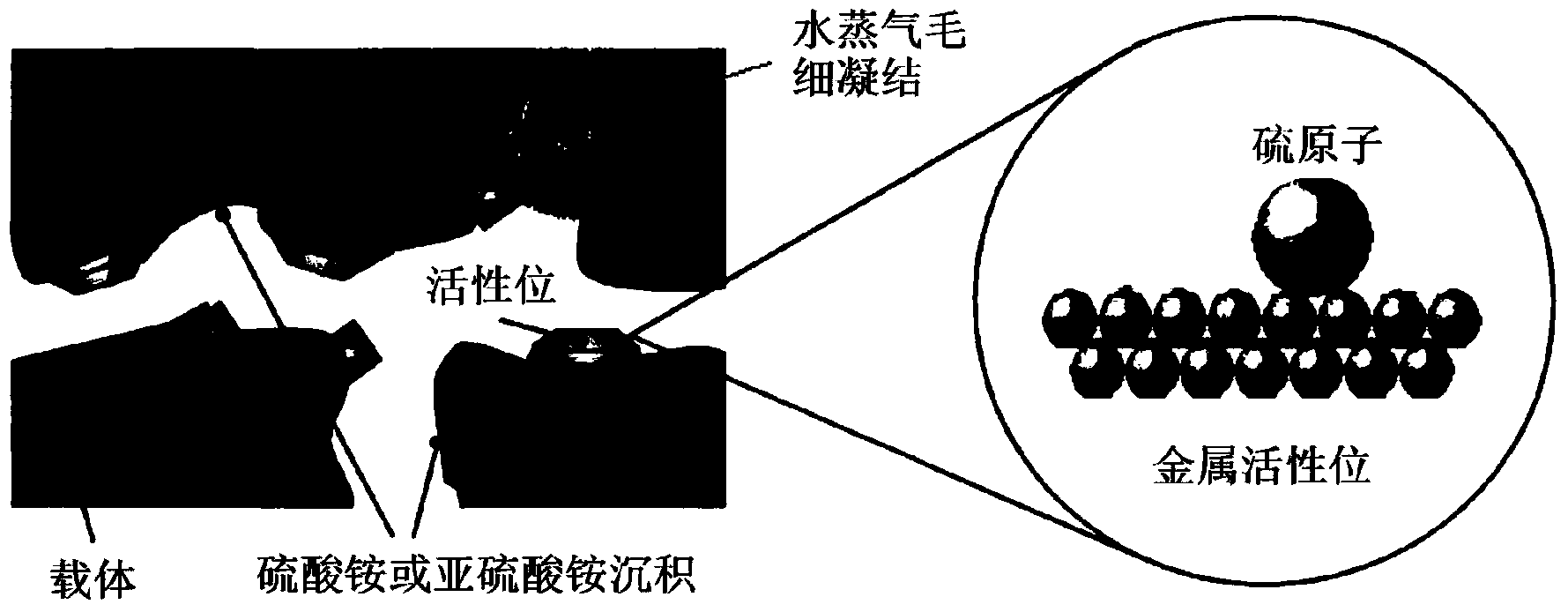
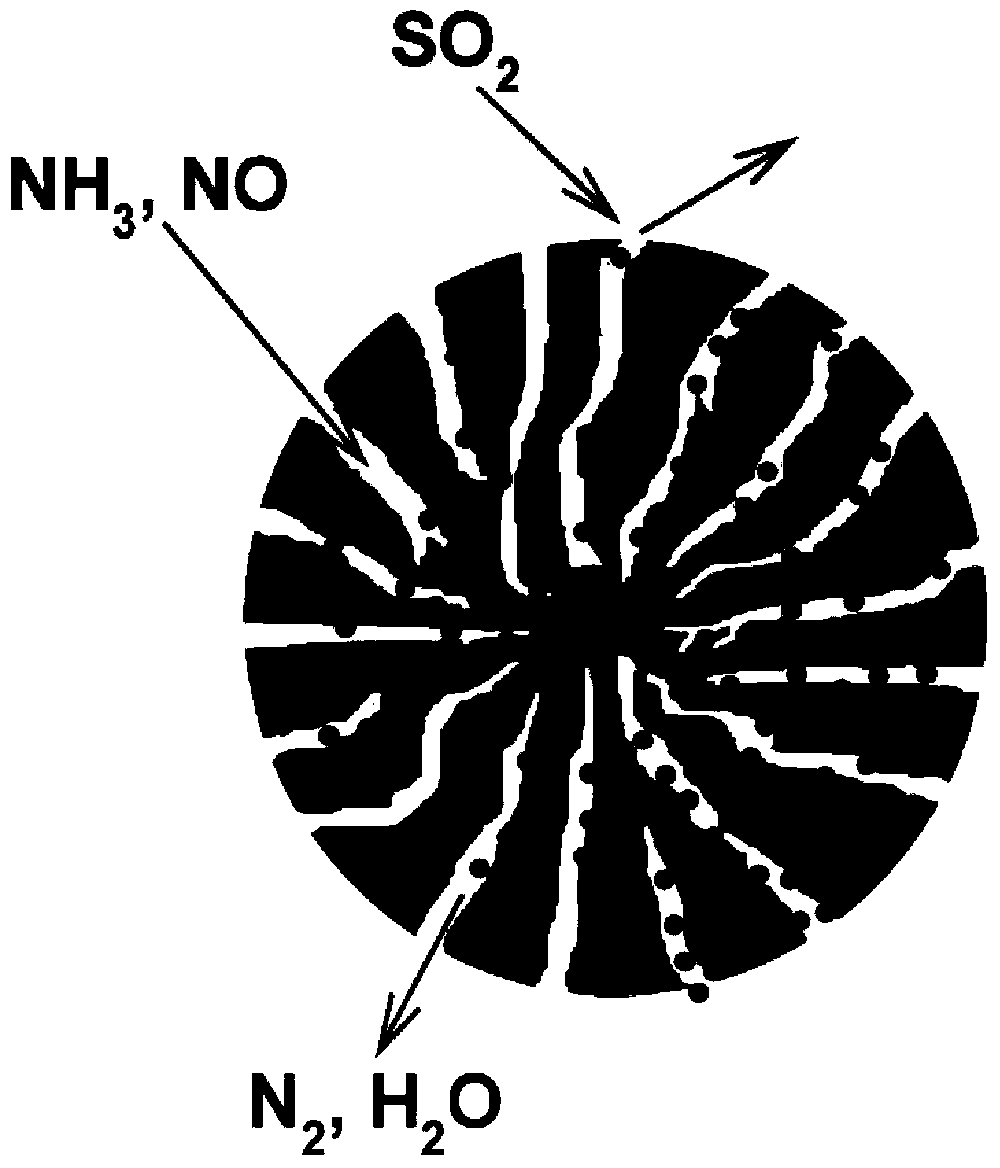
Low-temperature sulfur-resistant denitration catalyst and preparing method thereof
A denitrification catalyst and catalyst technology, applied in the direction of molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of nitrogen oxide pollution, not effectively controlled, weakened activity, etc., and achieve strong water resistance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] 10%Mn 0.4 Ce 0.6 o 2 / MS-3A
[0077] Its preparation process is as follows:
[0078] 1) Weigh a certain amount of manganese nitrate hydrate (Mn(NO 3 ) 2 ·xH 2 O), and cerium nitrate hexahydrate (Ce(NO 3 ) 3 ·6H 2 (0), and it is dissolved in an appropriate amount of deionized water to make a concentration of 50mM, a volume of A solution of 200mL, wherein the mol ratio of manganese ions and cerium ions is 4:6;
[0079] 2) Weigh 4g of 3A molecular sieve, and put it into the above-mentioned A solution;
[0080] 3) Transfer the suspension prepared in step 2) into a round bottom flask connected to a rotary evaporator and heat it to 80° C. under rotation, and the ion exchange reaction occurs continuously at this temperature for 5 hours;
[0081] 4) The above-mentioned reacted suspension was put to room temperature under the condition of natural cooling, and then the filter cake was obtained under the condition of vacuum filtration;
[0082] 5) The above-mentioned fi...
Embodiment 14
[0096] 95% Mn 0.4 Mg 0.6 o 2 5%TiO 2
[0097] Its preparation process is as follows:
[0098] 1) Weigh a certain amount of manganese nitrate hydrate (Mn(NO 3 ) 2 ·xH 2 O), and magnesium nitrate hexahydrate (Mg(NO 3 ) 2 ·6H 2 (0), and it is dissolved in an appropriate amount of deionized water to obtain a concentration of A solution that is 0.1M;
[0099] 2) Take a certain amount of citric acid solid particles, and dissolve it into an appropriate amount of deionized water to obtain a certain concentration of B solution; wherein, the concentration of citric acid in B solution and the total concentration ratio of metal ions in A solution are 1 :1;
[0100] 3) Pour solution B into solution A with an equal volume and stir continuously for half an hour to ensure that citric acid molecules and metal ions fully undergo chelation reactions;
[0101] 4) The reacted mixed solution was placed in a drying oven and evaporated and dried continuously at 80° C. for 12 hours to form...
Embodiment 28
[0120] 10% (95% Mn 0.4 Mg 0.6 o 2 5%TiO 2 ) / 90% AC
[0121] Its preparation process is as follows:
[0122] 1) Weigh a certain amount of activated carbon columnar particles at 30% H 2 / N 2 Continuous activation at 800°C for 1 hour under an atmosphere. After this step of hydrogenation treatment, the pore structure of activated carbon will be more developed, so as to facilitate the loading of denitrification active sites in the later stage;
[0123] 2) The above-mentioned activated carbon was placed in 1N hydrochloric acid and nitric acid aqueous solution and continued to soak for 8 hours. Wherein, the mol ratio of hydrochloric acid and nitric acid is 1:1;
[0124] 3) The activated carbon after the acidification treatment is repeatedly rinsed with deionized water at room temperature and under the condition of vacuum filtration until the filtrate becomes neutral;
[0125] 4) Place the cleaned activated carbon in an oven and dry at 105°C for 8 hours;
[0126] 5) The Mn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com