Method for determining oleaginous microalgae harvesting time by utilizing chlorophyll fluorescence parameter Pv/Fm
A technology of chlorophyll fluorescence and oil-producing microalgae, which is applied in the field of microalgae biology and bioenergy, can solve the problems of time-consuming and high reagent cost, accuracy interference, and interference with Raman spectral characteristics, so as to reduce time and reagent cost, Less interference factors and simple detection operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
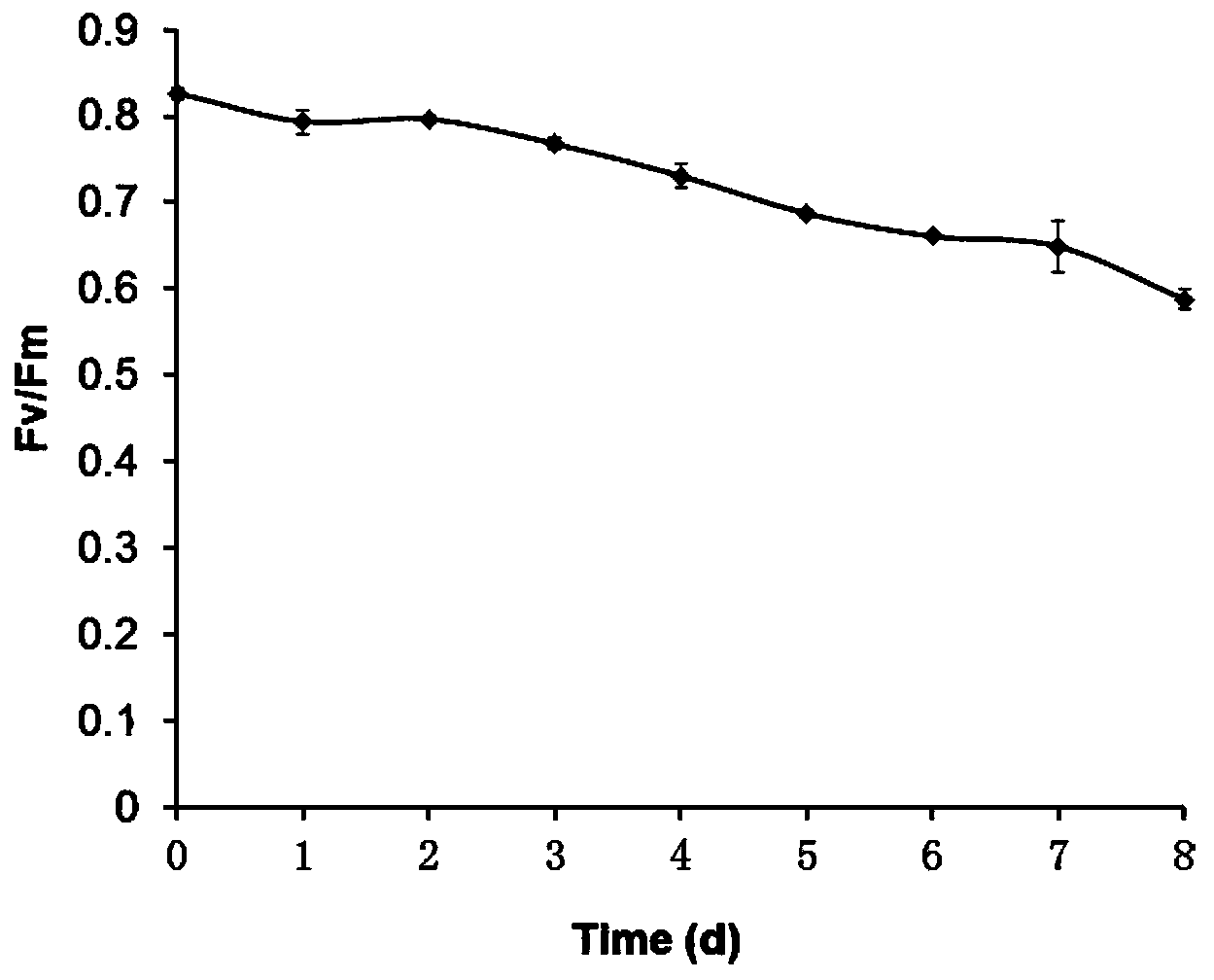
[0031] A method for determining the harvest time of oleaginous microalgae using the chlorophyll fluorescence parameter Fv / Fm, with a 500 mL culture scale of Chlorella sp.C2 (Chen et al., 2014, Plant & Cell Physiology, 55:634-644) as For example, the steps are:
[0032] 1. Autotrophic culture of oleaginous microalgae (Chlorella) and nitrogen deficiency stress treatment: add sterile BG11 liquid medium into a 1L sterile Erlenmeyer flask, the volume of the medium is 500mL. Then inoculate the purified Chlorella sp.C2 preserved in the laboratory and liquid-activated in BG11 liquid medium, and the inoculation density is OD 700 =0.05, at the same time at the temperature of 25℃ and the light intensity of 70μmol m -2 the s -1 Under the conditions of continuous light and aeration culture;
[0033] With 700nm as the scanning wavelength, establish a standard curve between OD value and biomass, and calculate the concentration of biomass in the liquid medium by measuring the OD value of t...
Embodiment 2
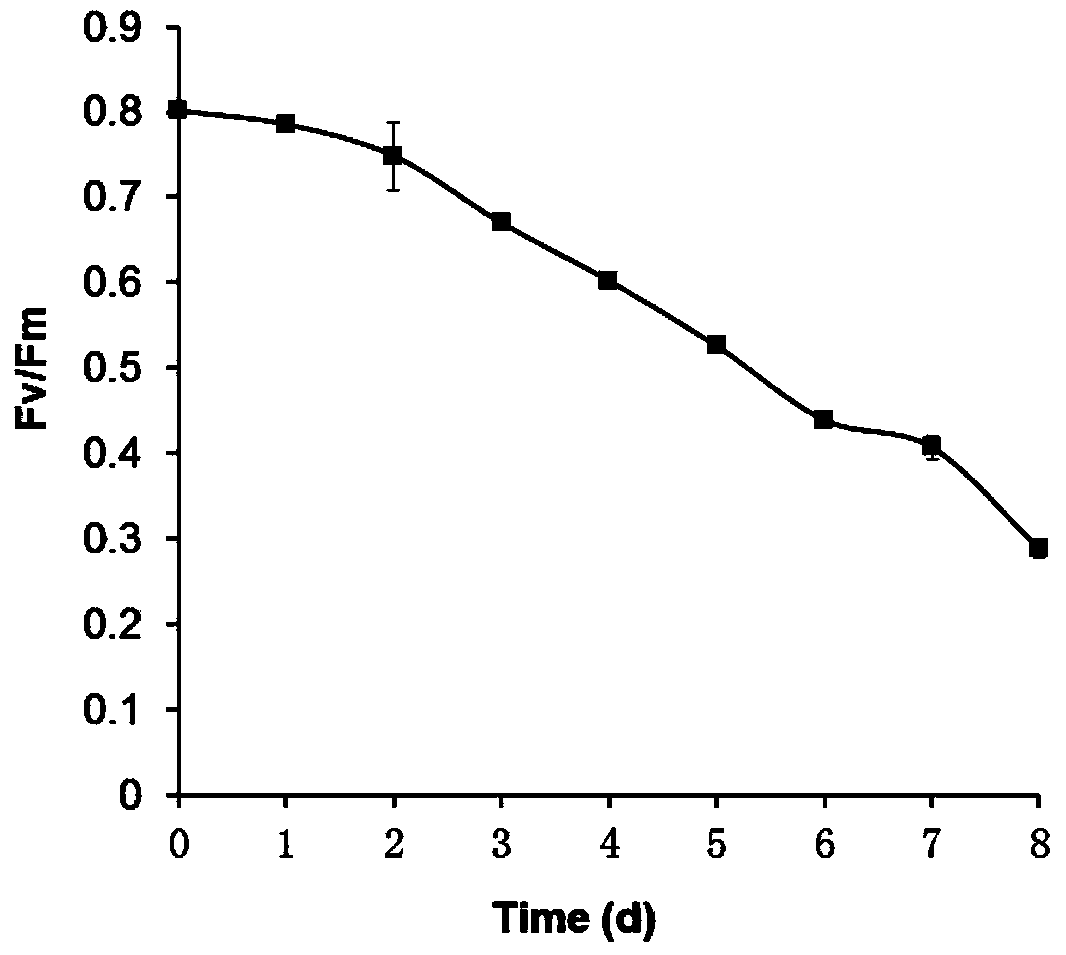
[0039] A method for determining the harvest time of oleaginous microalgae using the chlorophyll fluorescence parameter Fv / Fm, taking the 1L culture scale of Chlorella sorokiniana C3 (Zhang et al., 2013, PLoS ONE 8(7):e69225) as an example , whose steps are:
[0040] 1. Autotrophic culture and nitrogen deficiency stress treatment of any one of Chlamydomonas, Dunaliella, Nannochloropsis, Pseudochloropsis eyespots, Botrytis, Scenedesmus, Nitzki and Phaeodactylum tricornutum: The BG11 liquid medium of bacteria was added to a 2L sterile Erlenmeyer flask, and the volume of the medium was 1L. Then any one of the purified Chlamydomonas, Dunaliella, Nannochloropsis, Pseudochloropsis eyespots, Botrytis, Scenedesmus, Nitzschia and Phaeodactylum tricornutum that were preserved in the laboratory and performed liquid activation was inoculated in the In BG11 liquid medium, the seeding density is OD 700 =0.05, at the same time at the temperature of 25℃ and the light intensity of 70μmol m -...
Embodiment 3
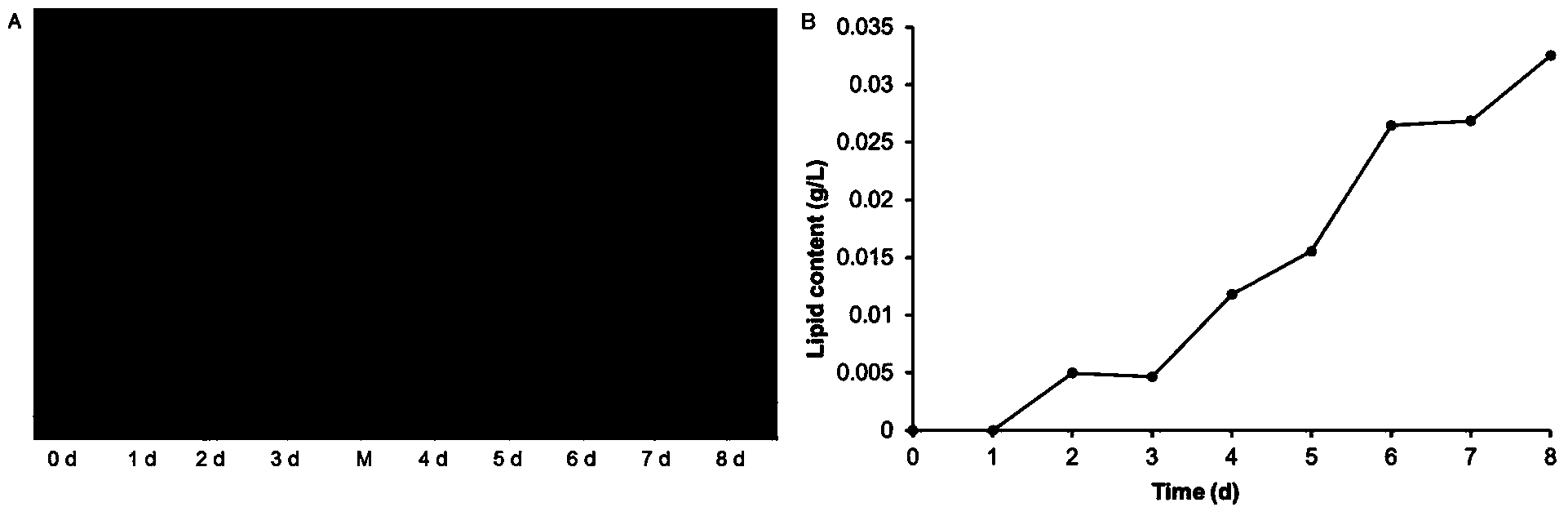
[0046] In order to verify the method and further highlight the superiority of the method, the applicant took Chlorella sp.C2 1L culture scale as an example, with the Fv / Fm recovery range 0.588-0.649 determined in Example 1, using chlorophyll Fluorescence parameter Fv / Fm determines the harvest time of oleaginous microalgae. At the same time, it is compared with the method of determining the harvest time of oleaginous microalgae by thin-layer chromatography analysis after oil extraction combined with image analysis software. A method using chlorophyll fluorescence parameter Fv / Fm to determine The method for harvesting time of oleaginous microalgae, the steps are:
[0047] 1. Chlorella autotrophic culture and nitrogen deficiency stress treatment: add sterile BG11 liquid medium into a 2L sterile Erlenmeyer flask, the medium volume is 1L. Then inoculate the purified Chlorella sp.C2 preserved in the laboratory and liquid-activated in BG11 liquid medium, and the inoculation density i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com