Compound having tyrosinase inhibitory activity and synthesis method of compound
A tyrosinase, inhibitory activity technology, used in the fields of food, cosmetics and medicine, can solve the problems of low selectivity, strong acid and strong base, too long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
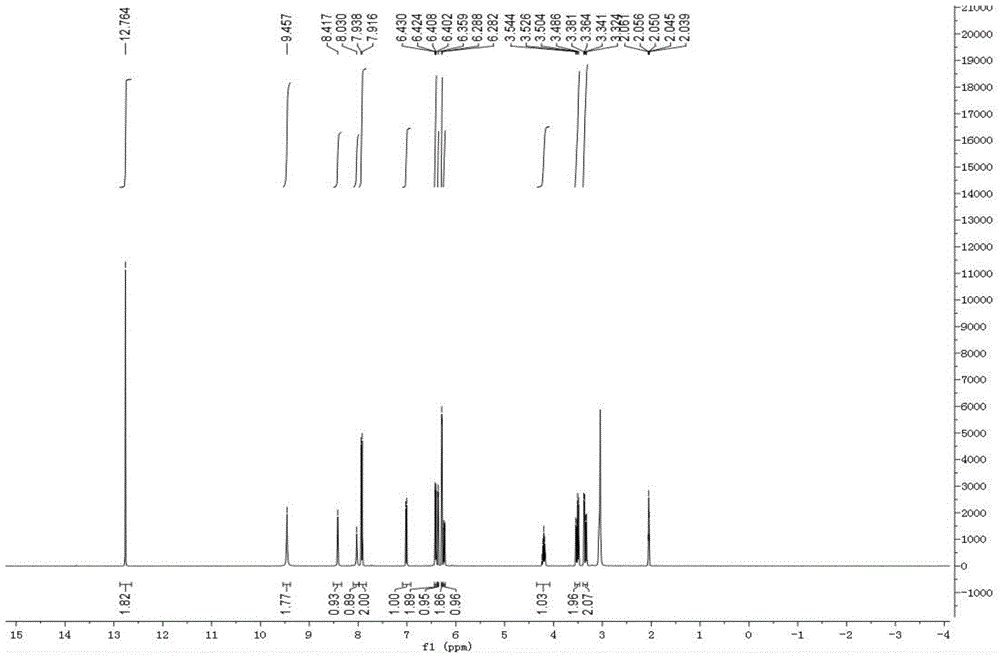
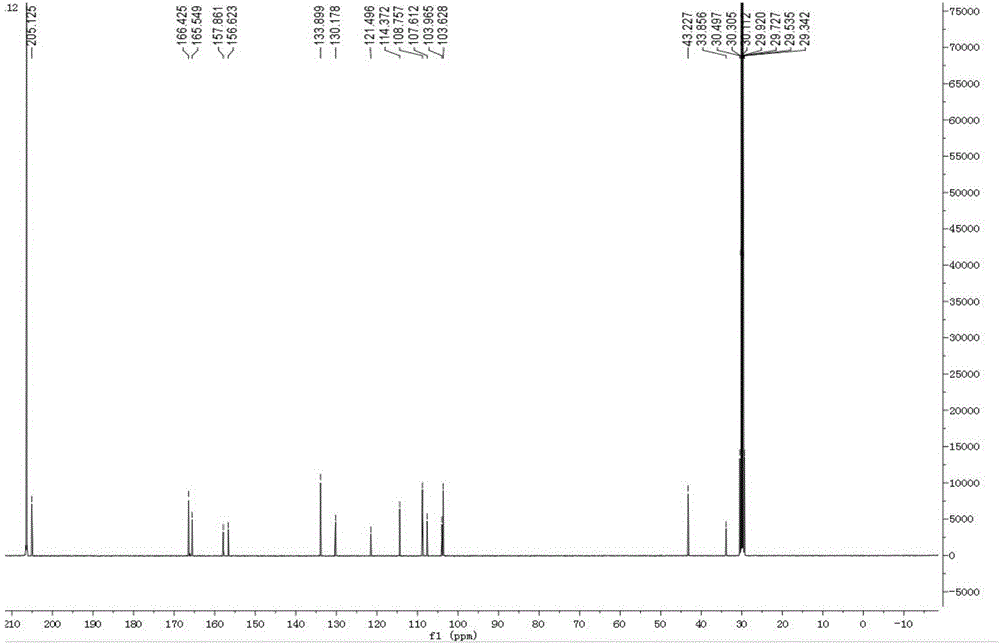
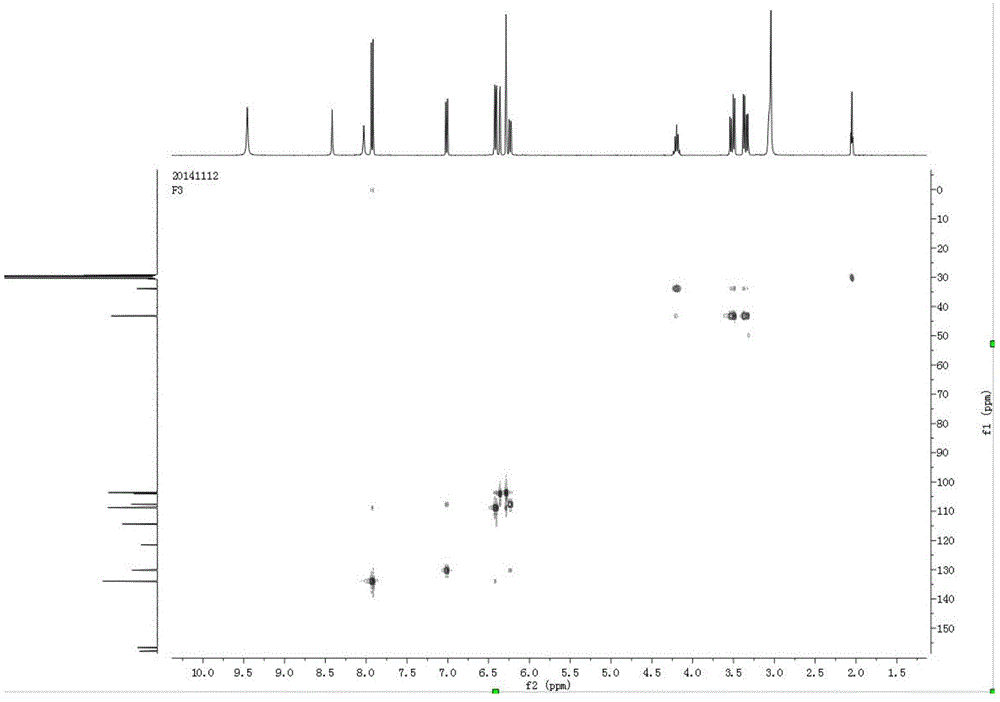
[0028] Example 1 Green Synthesis Method of 1,3,5-Tris-(2,4-dihydroxy-phenyl)-pentane-1,5-dione
[0029] Weigh 5g of 2,4-dihydroxybenzaldehyde, 2.5g of 2,4-hydroxyacetophenone, 2.1g of boric acid, 0.5g of ascorbic acid in a 50mL round bottom flask, add 25mL of PEG400 and stir to dissolve, place in a 120°C oil bath Stirred and refluxed for 6h, followed the progress of the reaction by TLC. The reaction solution was extracted three times with ethyl acetate, and the organic phase was concentrated to dryness under reduced pressure; the dried sample was eluted on a silica gel column (particle size 10-40μ) with dichloromethane:methanol=20:1 (v / v), and collected The eluate was concentrated and dried to obtain a dry sample; the dry sample was separated by gel column chromatography (Sephadex LH-20), and eluted with methanol-water (v / v, 1:1); Liquid chromatography (Waters preparative high performance liquid chromatograph, dual-wavelength detector, preparative column is YMC (250mm * 20mm,...
Embodiment 2
[0035] Example 2 Evaluation method for tyrosinase inhibitory activity
[0036] 1,3,5-tris-(2,4-dihydroxy-phenyl)-pentane-1,5-dione was dissolved in DMSO to prepare 50, 10, 5, 2.5, 0.5 μg / mL respectively. The positive control kojic acid was made into solutions with concentrations of 50, 25, 10, 5, and 2.5 μg / mL. Take 300 μL of each of the five concentrations of solutions, use 700 μL of pH 6.8 phosphate buffer solution to make 1 mL, add 0.1 mg / mL tyrosine 1 mL, and then add 1 mL of pH 6.8 phosphate buffer solution. Tyrosinase (400U / mL), incubate at 37°C for 20min, and measure the absorbance at 492nm.
[0037] Enzyme activity inhibition rate=[(A2-A1)-(B2-B1)] / (A2-A1)×100%
[0038] A1 is the absorption value without inhibitor at 0 min; A2 is the absorption value without inhibitor after 20 min;
[0039] B1 is the absorption value of inhibitor added at 0 min; B2 is the absorption value of inhibitor added after 20 min.
Embodiment 3
[0040] Example 3 Evaluation of compound tyrosinase inhibitory activity
[0041] IC of 1,3,5-Tris-(2,4-dihydroxy-phenyl)-pentane-1,5-dione inhibiting tyrosinase 50 The IC of kojic acid is 5.66 μM 50 IC of 67.6 μM, 2,4-dihydroxybenzaldehyde 50 >200 μM, IC of 2,4-dihydroxyacetophenone 50 >300 μM. The tyrosinase inhibitory activity of the compound 1,3,5-tris-(2,4-dihydroxy-phenyl)-pentane-1,5-dione is far greater than that of the positive control kojic acid and the reaction raw material 2,4-hydroxy Acetophenone and 2,4-dihydroxybenzaldehyde.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com