Serum-free cartilage cell culture solution
A technology of chondrocytes and culture medium, applied in the field of biomedical materials, can solve the problems of difficult clinical application of chondrocytes, accelerated dedifferentiation rate of chondrocytes, limited amount of cartilage tissue, etc., to maintain extracellular matrix secretion ability, good extracellular Matrix secretion ability and the effect of promoting cell function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
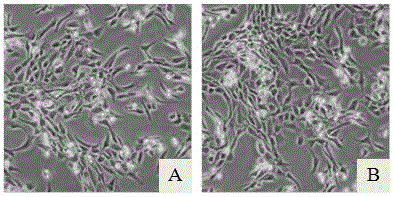
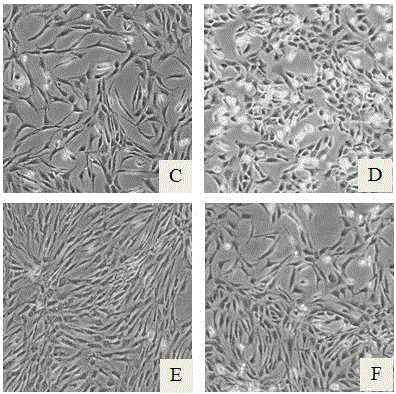
[0025] The obtained human cartilage tissue was obtained by enzymatic digestion to obtain primary chondrocytes and the cell viability and number were calculated. After washing with PBS, culture medium A was used respectively (culture medium A was a mixed culture medium of DMEM and F12 according to the volume ratio of 1:1, Then add 10% FBS) and the serum-free chondrocyte culture medium prepared in this example to resuspend, press 2 × 10 4 Pieces / cm 2 Inoculated into culture flasks, placed in an incubator at 37°C, 5% CO 2 Culture under conditions; after 48~72h, half of the medium was changed, and the culture was continued, and then the medium was changed every 48h. The adherence rate of primary human chondrocytes in serum-free group and serum group can reach about 60% within 48 hours, and subculture can be carried out when the confluence rate of chondrocytes reaches about 50%.
[0026] The serum-free medium used in this example is: the basal medium is a mixed medium of DMEM and...
Embodiment 2
[0029] The obtained human cartilage tissue was digested overnight by enzymatic digestion, and the primary chondrocytes were collected by centrifugation. After that, follow 0.5x10 4 / cm 2 Cell density was seeded into cell culture flasks at 37°C, 5% CO 2 The cells were cultured in a conditioned incubator; when the cells reached 60%-80% confluence, they were passaged by trypsin digestion. The first group was cultured with culture medium A, and the second group was cultured with the serum-free culture medium of this example. Observation was carried out when it was passed to the 3rd and 6th generations.
[0030] The serum-free medium used in this example is: the basal medium is a mixed medium of DMEM and F12 in a volume ratio of 1:1; 6g / L of dextran is added for stirring and dissolved, and then 0.5mL / L of lipid concentration is added respectively. agent, 75 mg / L sodium pyruvate, 0.1 μM T3, 0.05 mg / L hydrocortisone, 0.5 μg / L dexamethasone, 1 μg / L sodium selenite, 25 μM β-mercapto...
Embodiment 3
[0033] The obtained human cartilage tissue was digested by enzymatic digestion overnight, and primary chondrocytes were collected by centrifugation. 4 / cm 2 Cell density was seeded into cell culture flasks at 37°C, 5% CO 2 The cells were cultured in a conditioned incubator; when the cells reached 60%-80% confluence, they were passaged by trypsin digestion, and the serum-free medium of this example was used for culture.
[0034] The serum-free medium used in this example is: the basal medium is DMEM as the basal medium; 15g / L of dextran is added with stirring to dissolve, and then 0.1mL / L of lipid concentrate and 55mg / L of pyruvate are added respectively. Sodium, 0.5μM T3, 0.1mg / L Hydrocortisone, 5μg / L Dexamethasone, 2μg / L Sodium Selenite, 25μM β-mercaptoethanol, 100mg / L V C , 50 μg / mL glutamine, 25 μg / L bFGF, 10 μg / L EGF, 10 μg / L TGF-β, 5 μg / L PDGF, 5 μg / L BMP, 1 mg / L transferrin, 100 μg / L IGF, 125 μg / L GH, 20 nM progesterone, 75 μM butanediamine, 1% non-essential amino ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com