Comprehensive utilization method of silicate minerals
A silicate and mineral technology, used in the comprehensive utilization of non-metallic ores and hydrometallurgy, can solve the problems of harsh operation and equipment conditions, inability to prepare magnesium sulfate monohydrate products, and high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
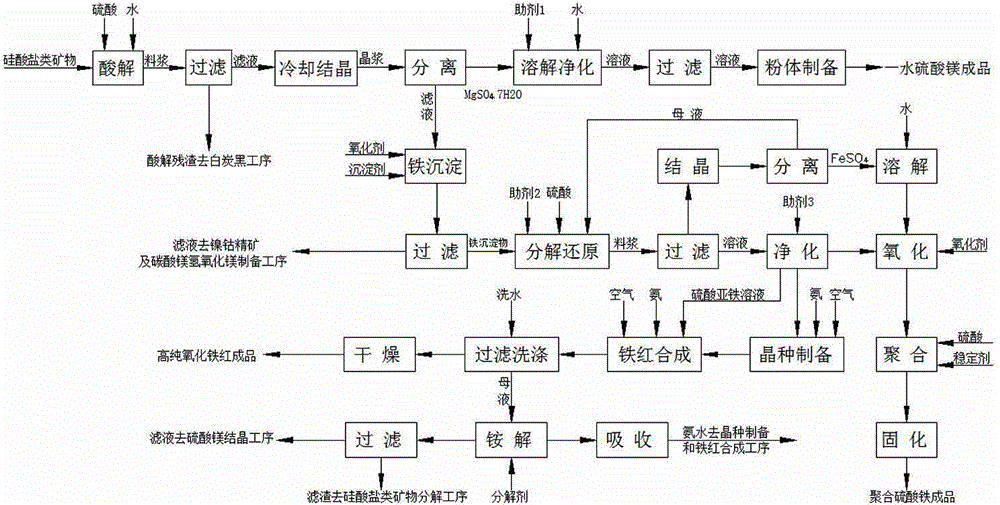
[0108] like figure 1As shown, the comprehensive utilization method of the silicate minerals, the processed ore is Sichuan Ya'an asbestos tailings, chemical composition: MgO: 35.74%, Fe 2 o 3 : 6.22%, SiO 2 : 41.81%, NiO: 0.37%, CoO: 0.031%, follow the steps below:
[0109] (1) Pulverize the asbestos tailings to 120 mesh, weigh 100kg of asbestos tailings powder and the secondary acid hydrolysis solution at a solid-to-liquid ratio of 1:2.5 (secondary acid hydrolysis solution 250L) and mix them in the primary acid hydrolysis reactor, After stirring evenly, add 1 kg of activator fluorite, then raise the temperature and add sulfuric acid, control the reaction temperature to 80°C, keep the reaction for 60 minutes, and control the end point pH value to 1. After the reaction was finished, 139kg of the first-level acidolysis residue (50.5% water content, 68.8kg in dry form) and 255L of the first-level acidolysis solution were obtained by filtration. MgSO in primary acid hydrolysis ...
Embodiment 2
[0124] The raw ore processed is asbestos tailings in Ya'an, Sichuan, chemical composition: SiO 2 : 41.81%, MgO: 35.74%, Fe 2 o 3 : 6.22%, NiO: 0.37%, CoO: 0.031%. Follow the steps below:
[0125] (1) Pulverize the asbestos tailings to 120 mesh, weigh 100kg of asbestos tailings powder and the secondary acid hydrolysis solution at a solid-to-liquid ratio of 1:5 (secondary acid hydrolysis solution 500L) and mix them in the primary acid hydrolysis reactor, After stirring evenly, add 1 kg of activator industrial calcium fluoride, then raise the temperature and add sulfuric acid, control the reaction temperature to 75°C, keep the reaction for 90 minutes, and control the end point pH value to 1. After the reaction was completed, 120 kg of the primary acidolysis residue (49.5% water content, 60.6 kg in dry form) and 500 L of the primary acidolysis solution were obtained by filtration. MgSO in primary acid hydrolysis solution 4 Concentration 213g / l, FeSO 4 The concentration was 2...
Embodiment 3
[0140] The raw ore processed is Serpentine Mine in Inner Mongolia Sunite Left Banner, chemical composition: MgO: 37.81%, SiO 2 : 36%, Fe 2 o 3 : 5.93%, Ni: 0.20%, Co: 0.009%.
[0141] (1) Crush the serpentine to 100 mesh, weigh 100kg of serpentine ore powder and the secondary acid hydrolysis solution at a solid-to-liquid ratio of 1:2.5 (secondary acid hydrolysis solution 250L) and mix them in the primary acid hydrolysis reactor After stirring evenly, add 1kg of activator, then raise the temperature and add sulfuric acid, control the reaction temperature to 85°C, keep the reaction for 60min, and control the end point pH value to 1.2. After the reaction was completed, 174 kg of the primary acidolysis residue (50.5% water content, 86 kg in dry form) and 262 L of the primary acidolysis solution were obtained by filtration. MgSO in primary acid hydrolysis solution 4 Concentration 432g / l, FeSO 4 The concentration was 42.7 g / l.
[0142] Mix the primary acidolysis residue and wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com