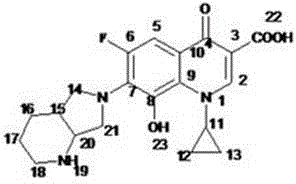
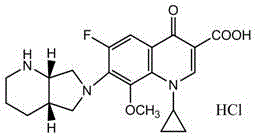
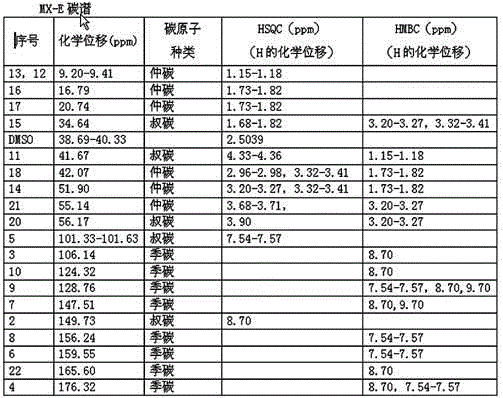
Method for preparing Moxifloxacin impurity E
A moxifloxacin and impurity technology, applied in the field of medicinal chemistry, can solve the problems of easy tar generation, long reaction time, degradation and damage, and achieve the effects of mild reaction conditions, shortened reaction time, and increased reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take moxifloxacin hydrochloride (30g, 0.068mol), dissolve it in 600mL of water, adjust the pH=7~8 with saturated sodium bicarbonate solution, then add dichloromethane (300ml×2) to extract the aqueous phase, and dichloromethane layer with anhydrous Dry over sodium sulfate for 1 h, filter, concentrate the filtrate to dryness, and dissolve the residue in 250 mL of acetonitrile.
[0035] Under the protection of nitrogen, hexamethyldisiloxane (17.8g, 0.11mol) and aluminum powder (3.8g, 0.14mol) were heated and stirred at a temperature of 60°C, solid iodine (28g, 0.11mol) was slowly added, and the temperature was raised to 140°C, reflux for 2 hours, slowly lower to room temperature, remove insoluble matter by filtration, and obtain about 42 g of freshly prepared crude iodotrimethylsilane oil. Under the protection of nitrogen, the obtained iodotrimethylsilane oil (42g, 0.21mol) was added to the moxifloxacin free base solution, sodium iodide (3g, 0.02mol) was added, and the rea...
Embodiment 2
[0038] Take moxifloxacin hydrochloride (20g, 0.05mol), dissolve it in 400mL of water, adjust the pH=7~8 with saturated sodium bicarbonate solution, then add ethyl acetate (300ml×2) to extract the aqueous phase, and wash the ethyl acetate layer with anhydrous Dry over sodium sulfate for 1 h, filter, concentrate the filtrate to dryness, and dissolve the residue in 200 mL of N,N-dimethylformamide DMF.
[0039] Under nitrogen protection, hexamethyldisiloxane (21.1g, 0.13mol) and aluminum powder (4.3g, 0.16mol) were heated and stirred at a temperature of 65°C, solid iodine (33g, 0.13mol) was slowly added, and the temperature was raised to Reflux reaction at 150°C for 2 hours, slowly lower to room temperature, remove insoluble matter by filtration, and obtain about 50 g of freshly prepared crude iodotrimethylsilane oil. Under the protection of nitrogen, the obtained iodotrimethylsilane oil (50g, 0.25mol) was added to the moxifloxacin free base solution, sodium iodide (4.5g, 0.03mol)...
Embodiment 3
[0042] Take moxifloxacin hydrochloride (50g, 0.12mol), dissolve it in 1000mL of water, adjust the pH=7~8 with saturated sodium bicarbonate solution, then add dichloromethane (300ml×2) to extract the aqueous phase, and dichloromethane layer with anhydrous Dry over sodium sulfate for 1 h, filter, concentrate the filtrate to dryness, and dissolve the residue in 300 mL of acetonitrile.
[0043] Under the protection of nitrogen, hexamethyldisiloxane (38.9g, 0.24mol) and aluminum powder (7.8g, 0.29mol) were heated and stirred at a temperature of 62°C, and solid iodine (60.9g, 0.24mol) was slowly added, and the temperature was raised To 145°C, reflux for 2 hours, slowly lower to room temperature, remove insoluble matter by filtration, and obtain about 96 g of freshly prepared crude iodotrimethylsilane oil. Under the protection of nitrogen, the obtained iodotrimethylsilane oil (96g, 0.48mol) was added to the moxifloxacin free base solution, sodium iodide (9g, 0.06mol) was added, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com