Super-paramagnetic iron oxide nanoparticle compound as well as preparation method and application thereof
An iron oxide nano-particle complex technology, applied in the fields of nano-drugs, nano-technology, nano-technology, etc., can solve the problems of the degree of progression of difficult kidney disease, dynamic evaluation of treatment effect, massive bleeding, sepsis, and unacceptable patients, etc. The effect of low production cost, simple production process and uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 superparamagnetic iron oxide nanoparticles
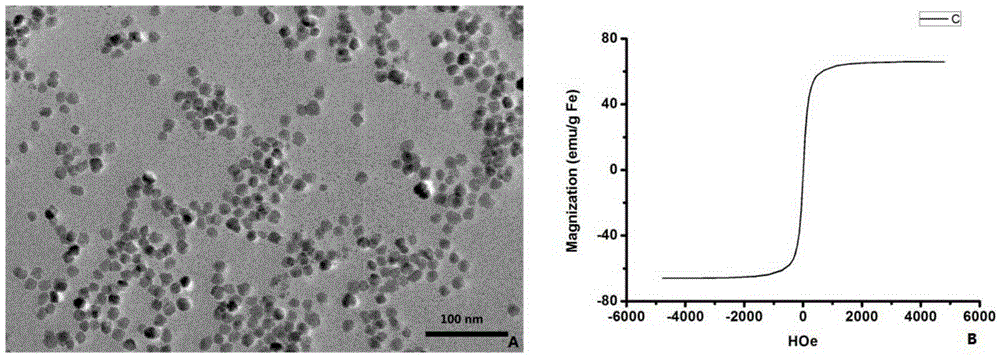
[0027] Add 0.9 mmol of iron triacetylacetonate and 0.9 mmol of carboxymethylated methoxypolyethylene glycol to 20 ml of analytically pure 2-pyrrolidone solution, and blow nitrogen gas for 30 minutes. After completion, the mixture was heated at 200°C for 30 minutes, then changed to 240°C for 40 minutes. After the reaction, the mixed product was cooled at room temperature, and 100 ml of diethyl ether / acetone (volume ratio 5:1) mixed solution was slowly added until precipitation was precipitated. After centrifugation, the precipitated black precipitate was washed with pure water and vacuum-dried to obtain superparamagnetic iron oxide nanoparticles with a particle size of 25.3±1.5nm. TEM images and magnetic saturation images ( figure 1 ) showed that the nanoparticles were fine-grained and uniform in size ( figure 1Middle A); The saturation magnetization is 66.02emu / mg ( figure 1 Middle B).
Embodiment 2
[0028] Example 2 Synthesis of MR Molecular Imaging Probes
[0029] Take 1 mg of the superparamagnetic iron oxide nanoparticles obtained in Example 1 (calculated based on iron content), add boric acid buffer solution (pH value 9, 0.5 ml), and shake at room temperature (25° C.) for 10 min. Then add 1 mg 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC HCL), 0.5 mg N-hydroxysulfosuccinimide (NHS), shake at 37 ° C for 30 min , adding 0.2 mg of rabbit anti-rat anti-IL-1 antibody (purchased from Beijing Boaosen) and gently shaking at 37° C. for 3 h. After the end, rinse the tube wall with phosphate buffer (pH 7.4), dilute the above liquid in phosphate buffer, and use an ultrafiltration centrifuge tube to centrifuge three times at 4°C and 3000 rpm for 30 minutes each time to remove unbound free antibody. After centrifugation, use a pipette gun to suck out the precipitate and dissolve it in a phosphate buffer with a pH value of 7.4, and adjust the concentration to 1 m...
Embodiment 3
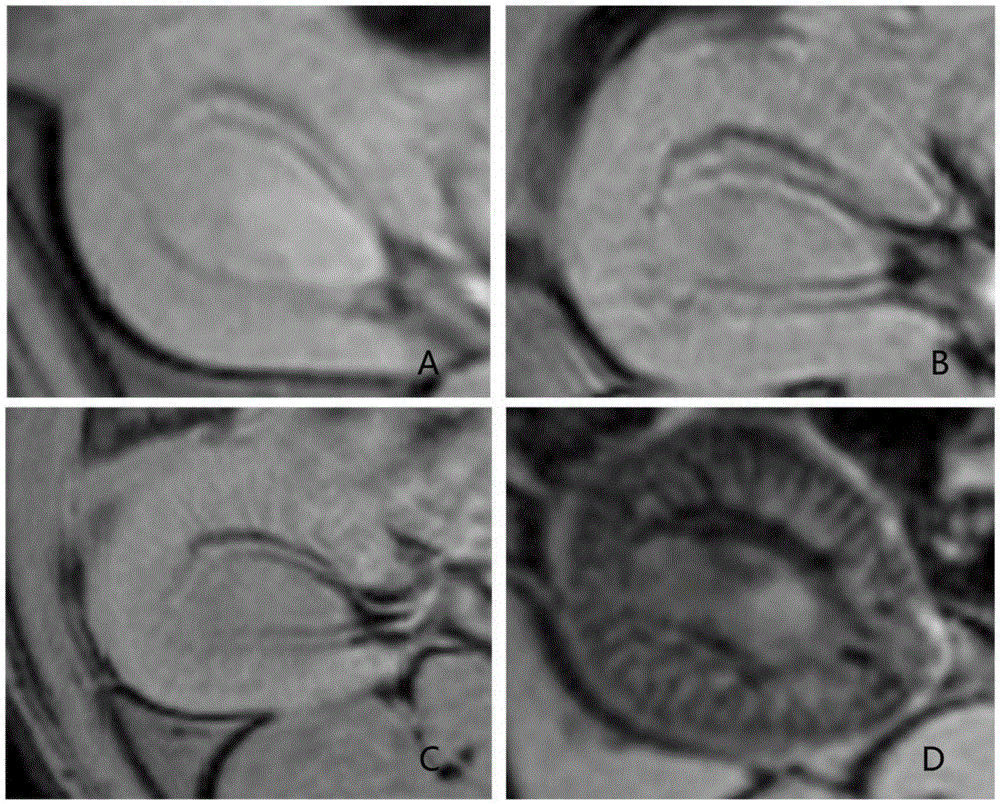
[0030] Example 3 MR Molecular Imaging of Normal Rats and Chronic Glomerulonephritis Rats
[0031] The rat model of mesangial proliferative nephritis adopts the anti-Thy-1 antibody induction method commonly used in the world, and the modeling process is as follows: male rats are injected with anti-Thy-1 monoclonal antibody OX-7 (1mg / kg) separately through the tail vein, On the 7th day, the model was successfully produced. Then normal male SD rats and male mesangial proliferative nephritis rat models were used for in vivo MR imaging. The MR imaging system is German Bruker7.0Tmicro-MR, the inner diameter of the horizontal scanning frame is 16cm, and the rat head coil of 38mm is used. After being anesthetized with 4% isoflurane, the mice were placed in a plexiglass scanning bed, and the brains of the mice were fixed with braces and ear bars. Anesthesia was maintained with 1.5% isoflurane: air mixture, and heart rate and respiration were monitored. The scanning range covers both...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com