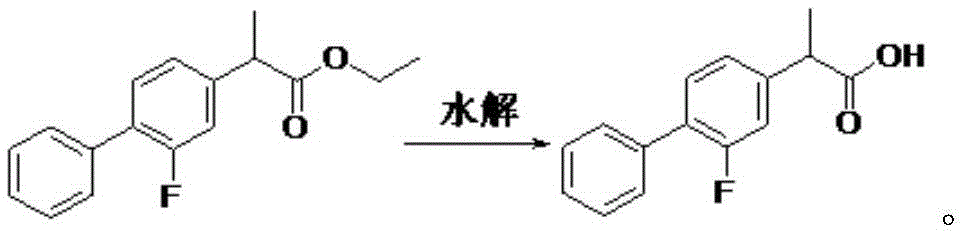
Preparation method of 2-(2--fluoro-4-biphenylyl) propionic acid
A biphenyl and propionic acid technology, applied in the preparation of carboxylate, the preparation of organic compounds, the preparation of carboxylate/lactone, etc., can solve the complex process of flurbiprofen, poor environmental safety, long reaction time, etc. problems, to achieve the effect of high product yield, high equipment utilization, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
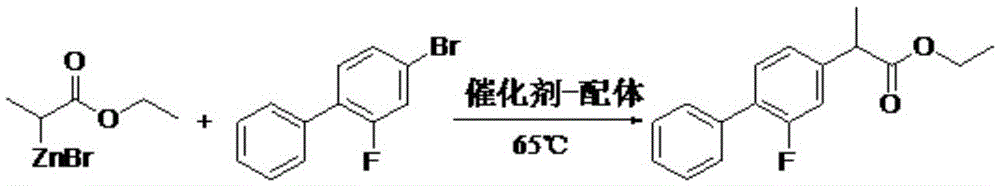
[0024] The preparation steps of 2-(2-fluoro-4-biphenyl)propionic acid are as follows:
[0025] 1) Preparation of zinc reagent
[0026] Under the protection of nitrogen, 100g of zinc powder, 20g of trimethylsilylchloride and 100g of tetrahydrofuran were successively added to the reaction flask, stirred at room temperature for 15min, heated to reflux (temperature 65°C), and slowly added dropwise with ethyl 2-bromopropionate 500g of tetrahydrofuran solution (containing 200g of ethyl 2-bromopropionate, dripped over 2h), reflux at 65°C for 3.5h to obtain the zinc reagent for later use; the reaction formula is as follows:
[0027]
[0028] 2) Synthesis of ethyl 2-(2-fluoro-4-biphenyl)propionate
[0029] Add 206 g of 4-bromo-2-fluorobiphenyl, 3.1 g of nickel diacetylacetonate, 7.2 g of triphenylphosphine, and 731 g of tetrahydrofuran in the reaction flask, stir and mix at room temperature, and slowly add the above zinc reagent dropwise under nitrogen protection ( 2h), heat and r...
Embodiment 2
[0036] The preparation steps of 2-(2-fluoro-4-biphenyl)propionic acid are as follows:
[0037] 1) Preparation of zinc reagent
[0038] Under the protection of nitrogen, add 200g of zinc powder, 60g of trimethylchlorosilane, and 400g of tetrahydrofuran to the reaction flask in sequence, stir at room temperature for 15min, heat to reflux (temperature 66°C), and slowly add dropwise the solution of ethyl 2-bromopropionate 1100g of tetrahydrofuran solution (containing 500g of ethyl 2-bromopropionate, dripped in 2.5h), reflux at 66°C for 4h to obtain the zinc reagent for use;
[0039] 2) Synthesis of ethyl 2-(2-fluoro-4-biphenyl)propionate
[0040] Add 440g of 4-bromo-2-fluorobiphenyl, 11g of nickel chloride, 22g of terpyridine, and 1980g of tetrahydrofuran into the reaction flask in sequence, stir and mix well at room temperature, and slowly add the above zinc reagent dropwise under nitrogen protection (2.5h to finish) , heated under reflux at 60°C for 4 hours, after the reaction...
Embodiment 3
[0045] The preparation steps of 2-(2-fluoro-4-biphenyl)propionic acid are as follows:
[0046] 1) Preparation of zinc reagent
[0047] Under the protection of nitrogen, 350g of zinc powder, 35g of trimethylsilyl chloride, and 500g of tetrahydrofuran were successively added to the reaction flask, stirred at room temperature for 20min, heated to reflux (temperature 70°C), and slowly added dropwise with ethyl 2-bromopropionate 1950g of tetrahydrofuran solution (containing 1050g of ethyl 2-bromopropionate, dripped over 3h), reflux at 70°C for 3h to obtain the zinc reagent for later use;
[0048] 2) Synthesis of ethyl 2-(2-fluoro-4-biphenyl)propionate
[0049] Add 720g of 4-bromo-2-fluorobiphenyl, 21.6g of 1,2-bis(diphenylphosphine)ethane nickel chloride, 43.2g of triphenylphosphine, and 4200g of tetrahydrofuran in the reaction flask, and stir and mix at room temperature. Evenly, slowly add the above-mentioned zinc reagent dropwise under the protection of nitrogen (3h), heat and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com