Preparation method of perhydrofluorene or perhydrofluorene alkyl substitute
一种氢芴烷基、取代物的技术,应用在高能量密度燃料合成领域,能够解决衍生物条件苛刻、成本高、卤素分离回收困难等问题,达到原料来源绿色、易规模化生产、操作流程复杂的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 25.2g of anisole, 3.08g of dibenzyl ether, and 0.1g of MMT-K10 into a three-necked flask with a volume of 50mL, stir magnetically, and react at a temperature of 110°C for 2 hours; transfer the above reaction mixture to a distillation apparatus for decompression Purified by distillation, and collected fractions at a temperature of 160-210°C at -0.08 MPa to obtain 6 g of colorless organic fractions.
[0028] Use gas chromatography to analyze the alkylation reaction product of preparing diphenylmethane derivatives, dibenzyl ether conversion rate 100%, anisole conversion rate 15%, diphenylmethane derivatives (i.e. substituted or unsubstituted diphenylmethane ) Yield 90%. After distillation under reduced pressure, pure diphenylmethane or a mixture of diphenylmethane with substituents can be obtained.
Embodiment 2-18
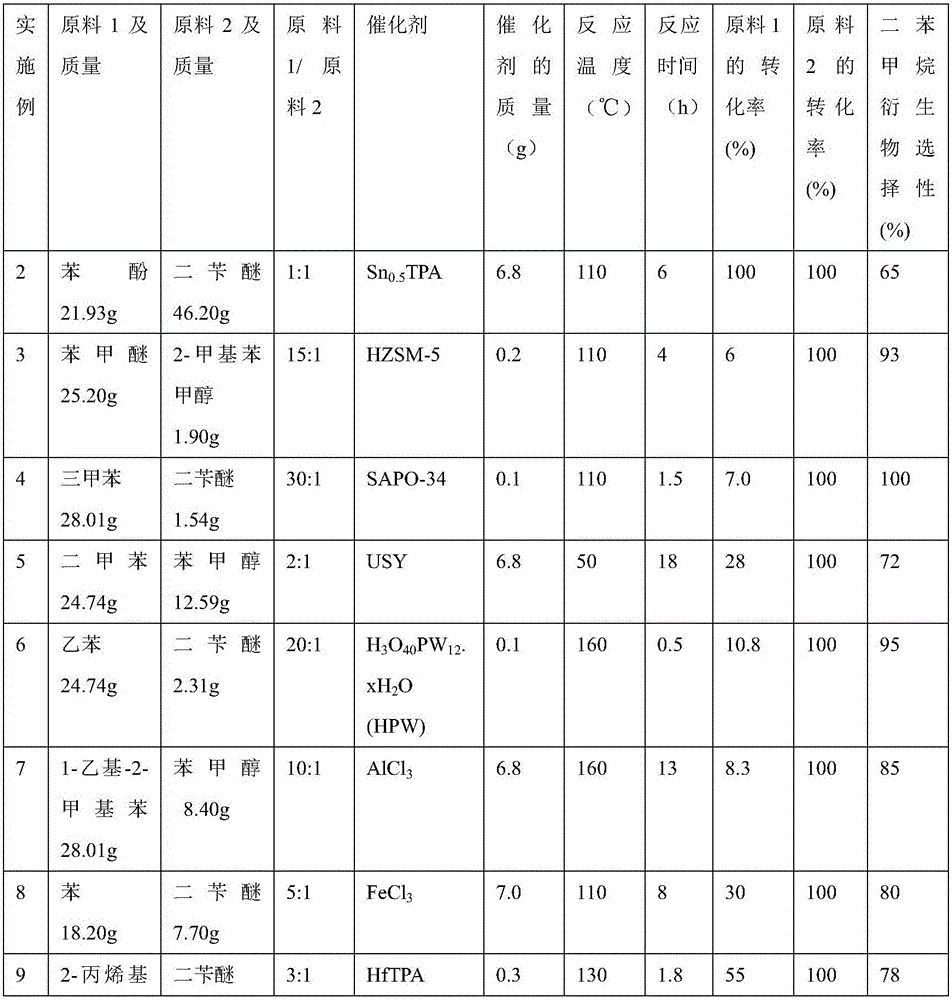
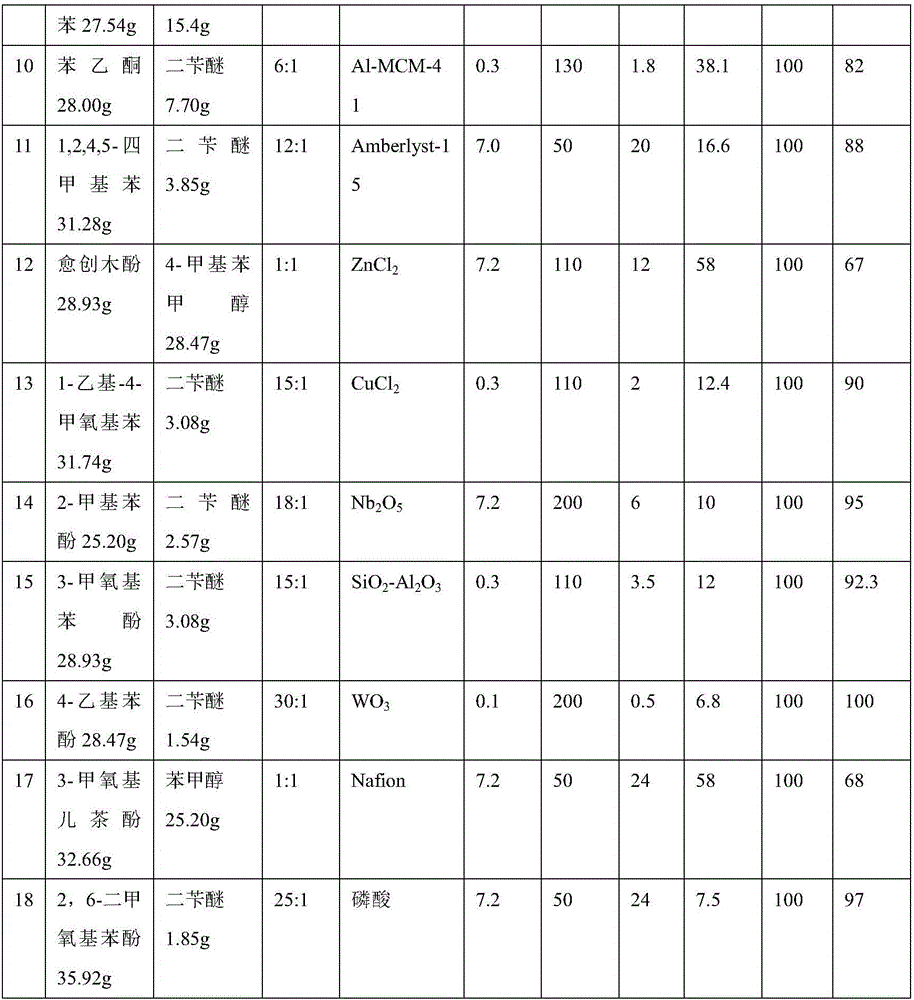
[0030] Same as embodiment 1, SiO 2 -Al 2 o 3 , HZSM-5, Al-MCM-41, Hβ, MMT-K10, SAPO-34, USY, H 3 o 40 PW 12· wxya 2 O(HPW), Amberlyst-15, Nafion, AlCl 3 , FeCl 3 , ZnCl 2 , CuCl 2 , metal-modified HPW (such as Sn 0.5 TPA, HfTPA), Nb 2 o 5 、WO 3 Such metal oxides and liquid phosphoric acid can respectively catalyze lignin containing benzene ring derivatives and benzyl compounds (2-methylbenzyl alcohol, 4-methylbenzyl alcohol, isomers of 2-methylbenzyl alcohol, benzene Alkylation reaction of methanol and benzyl alcohol dehydration condensation product (dibenzyl ether, etc.). Now the results of the yield of catalyst and its consumption, reactant conversion rate, reactant ratio, temperature of reaction, reaction time, and diphenylmethane derivative (i.e. substituted or unsubstituted diphenylmethane) are listed in Table 1:
[0031] Table 1
[0032]
[0033]
[0034] It can be seen from the above Examples 1-18 that lignin derivatives (phenols or aromatic hydroca...
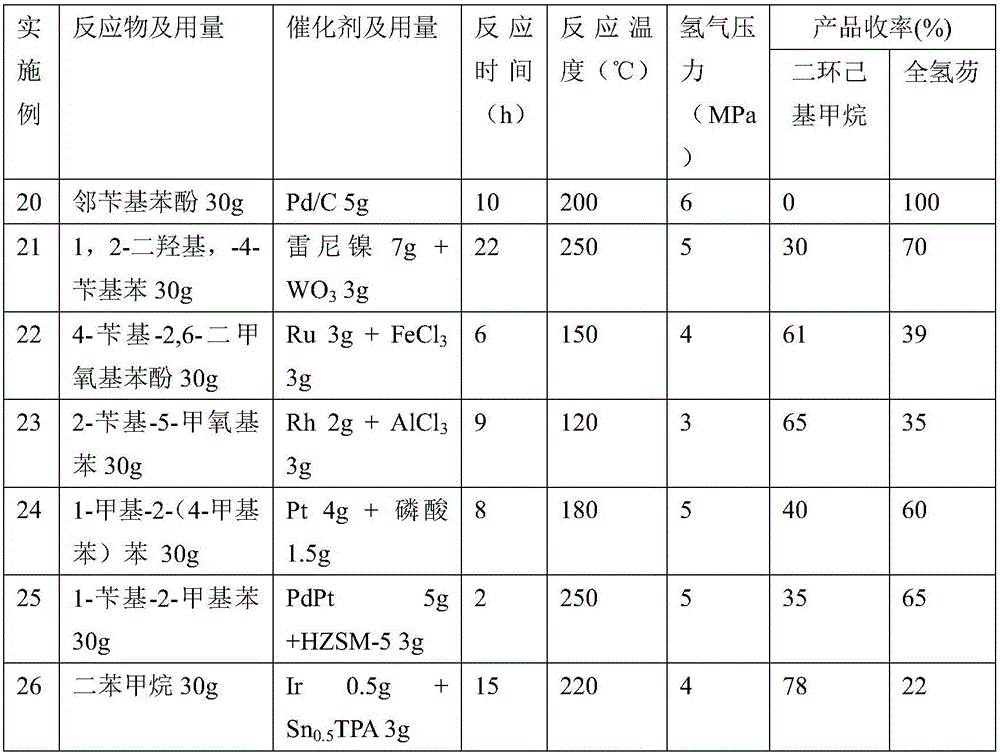
Embodiment 19
[0036] Adding 30g o-benzyl anisole, 3.6g palladium carbon, and 3gHZSM-5 catalyst into a 100mL pressure reactor with stirring, wherein the mass percentage of palladium in the palladium carbon is 5wt%, and the stirring rate is 680 rev / min, under the conditions of hydrogen pressure of 6 MPa and temperature of 200° C. for 10 hours, and centrifugation to obtain 26 g of colorless organic liquid.
[0037] The prepared fuel was analyzed by GC-MS analysis and the product was composed of two components (including a mixture of 35% dicyclohexylmethane and 65% perhydrofluorene in yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com