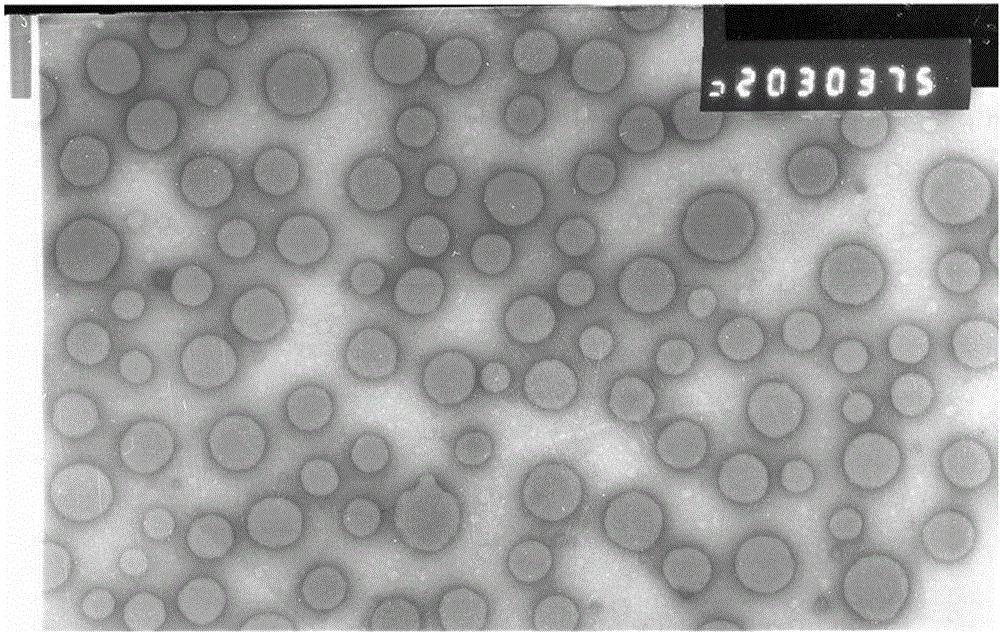
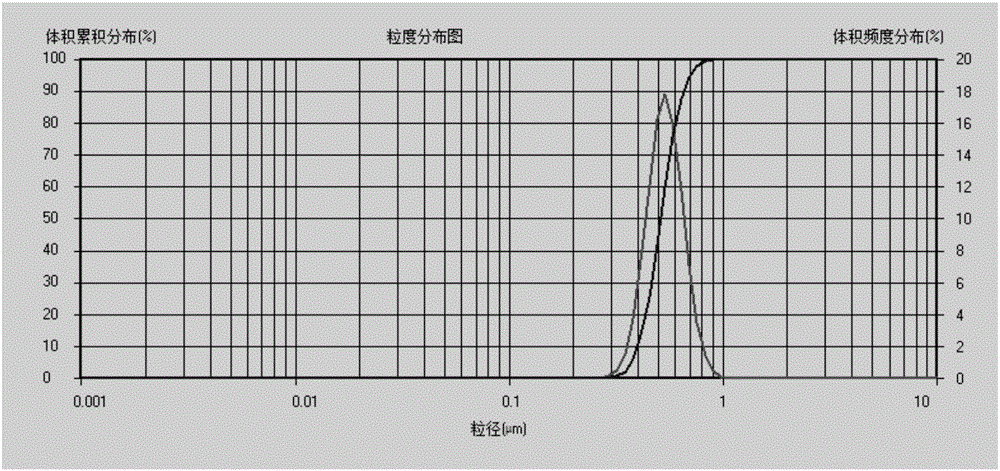
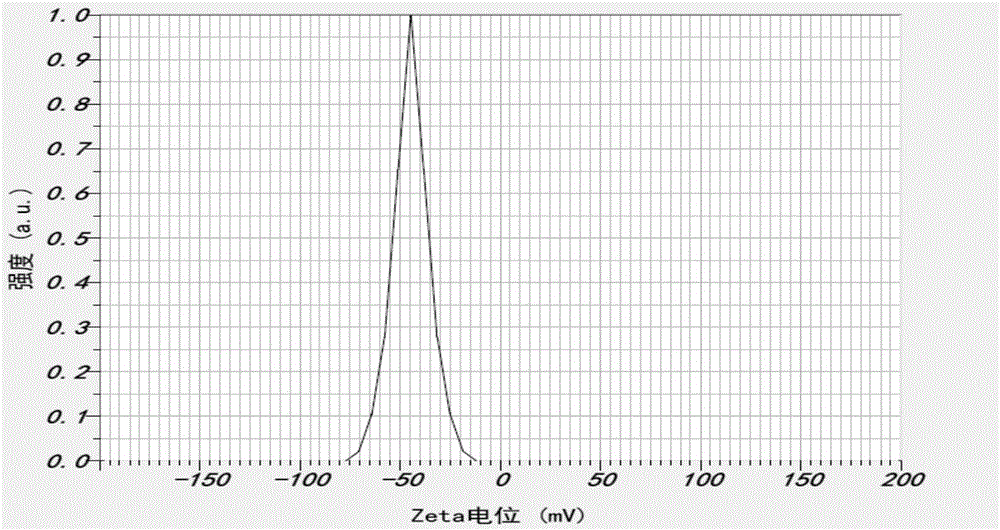
Enrofloxacin nanosuspension and preparation method thereof
A nano-suspension and enrofloxacin technology, which is applied in liquid delivery, pharmaceutical formula, emulsion delivery, etc., can solve the problem of low bioavailability of solid granules, poor water solubility of enrofloxacin, and low drug content in emulsions, etc. problem, to achieve the effect of improving bioavailability, improving absorption and bioavailability, and high drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of enrofloxacin nano-suspension, the preparation method of this enrofloxacin nano-suspension comprises the steps:
[0034] 1) Weigh 4.2 g of enrofloxacin bulk drug and 0.1 g of hydroxyethyl starch sodium and dissolve them in 25 mL of sodium hydroxide solution (pH=8.1) to form a good solvent A;
[0035] 2) Weigh 0.65g of Poloxamer 188, 0.56g of Gelucire44 / 14, and 0.50g of polyethylene glycol 400 and dissolve them in 75mL of aqueous hydrochloric acid (pH=2.5) to form poor solvent B;
[0036] 3) Control the temperature at 10°C, drop the above-mentioned good solvent A into the poor solvent B at a dropping speed of 5mL / min, and control the ultrasonic frequency to 45KHz at the same time, and disperse with ultrasonic stirring. After the dropping is completed, continue ultrasonic dispersion for 0.5h, A primary nanosuspension with blue opalescence is formed;
[0037] 4) Put the obtained primary nanosuspension in a high-pressure homogenizer, and circulate it 5 times under...
Embodiment 2
[0040] A kind of enrofloxacin nano-suspension, the preparation method of this enrofloxacin nano-suspension comprises the steps:
[0041] 1) Weigh 4.5g of enrofloxacin bulk drug and 0.1g of sodium hydroxyethyl starch, dissolve in 25mL of sodium hydroxide solution (pH=8.2) to form good solvent A;
[0042] 2) Weigh 0.85g soybean lecithin and 0.60g Labrasol, dissolve in 75mL phosphoric acid aqueous solution (pH=2.1) to form poor solvent B;
[0043] 3) Control the temperature at 8°C, drop the above-mentioned good solvent A into the poor solvent B at a rate of 2.5mL / min, and control the ultrasonic frequency to 45KHz at the same time, and disperse with ultrasonic stirring. A primary nanosuspension with blue opalescence is formed;
[0044] 4) Put the obtained initial nano-suspension in a high-pressure homogenizer, and circulate 10 times under a pressure of 500 bar to obtain the enrofloxacin nano-suspension;
[0045]The enrofloxacin nanosuspension was detected by a laser nanometer pa...
Embodiment 3
[0047] A kind of enrofloxacin nano-suspension, the preparation method of this enrofloxacin nano-suspension comprises the steps:
[0048] 1) Weigh 4.05g of enrofloxacin bulk drug and 0.15g of hydroxyethyl starch sodium and dissolve in 25mL of phosphate buffer (pH=8.5) to form good solvent A;
[0049] 2) Dissolve 0.82g of Labrasol and 0.49g of polyethylene glycol 400 in 75mL of phosphoric acid aqueous solution (pH=2.8) to form poor solvent B;
[0050] 3) Control the temperature at 2°C, drop the above-mentioned good solvent A into the poor solvent B at a dropping speed of 10mL / min, and control the stirring speed at 200r / min at the same time, stir and disperse with a high-shear emulsifier, and the dropping is completed. Continue stirring for 2h to form an initial nanosuspension;
[0051] 4) Put the gained initial nano-suspension in a high-pressure homogenizer, and circulate it 4 times under 1000 bar pressure to obtain the enrofloxacin nano-suspension;
[0052] The enrofloxacin n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com