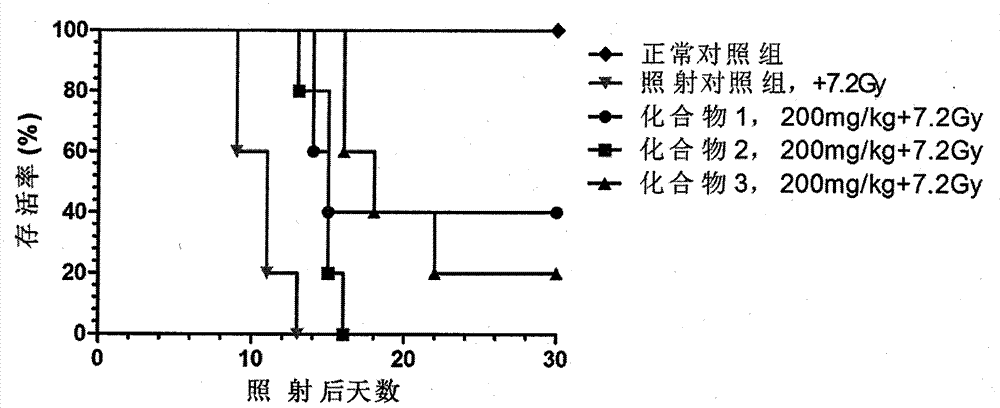
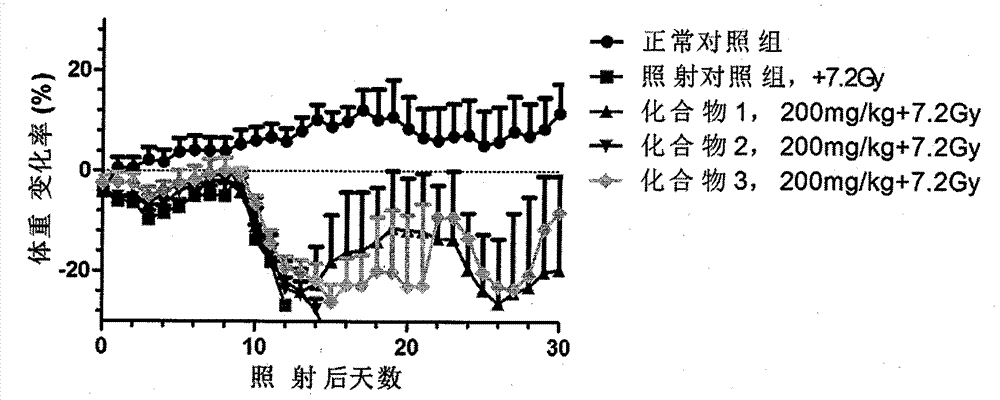
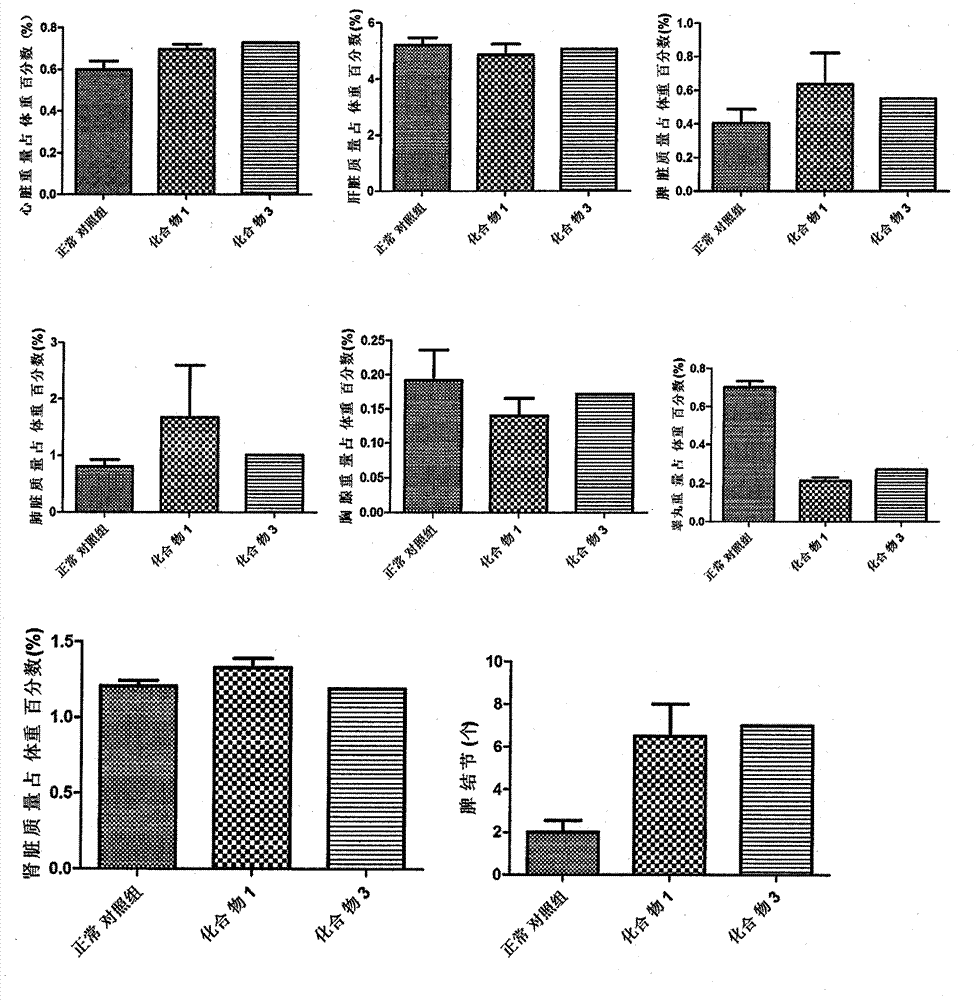
Novel compounds with radioprotective effects and preparation method and pharmaceutical use thereof
A technology of flavonoids and compounds, applied in the field of medicine, can solve the problems of limiting the wide application of radiotherapy, short half-life, and high price, and achieve the effects of prolonging the survival period and survival rate of animals, alleviating side effects of radiotherapy, and reducing biological damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The preparation method of general formula (I) compound:
[0063]
Embodiment 1
[0064] Example 1: 4-(3,7-bis((2,2-dimethylthiazole-3-carbonyl)oxy)-5-hydroxy-4-oxo-4hydro-benzopyran-2-yl) -Synthesis of 1,2-phenylbis((2,2-dimethylthiazole-3-carbonyl) (compound 1)
[0065] Step 1: Synthesis of 2,2-dimethylthiazolidine-3-carbonyl chloride
[0066]
[0067] 2,2-Dimethylthiazole (1.17g, 10.0mmol) was dissolved in anhydrous dichloromethane (40ml), triphosgene (1.50g, 5mmol) was added in an ice-water bath, and then DIPEA (2.58g, 20.0 mmol). Remove the ice-water bath and stir at room temperature for half an hour. After the reaction, water (20ml) was added, extracted with dichloromethane (30ml×3), and the combined organic phases were washed with saturated brine (20ml). Drying in vacuo gave a crude orange oil (1.8 g, 99%) which was used directly in the next step.
[0068] Step 2: 4-(3,7-Bis((2,2-dimethylthiazole-3-carbonyl)oxy)-5-hydroxy-4-oxo-4hydro-benzopyran-2-yl)- Synthesis of 1,2-phenylbis((2,2-dimethylthiazole-3-carbonyl)(compound 1)
[0069]
[00...
Embodiment 2
[0071] Example 2: 2-(3,4-bis((2,2-dimethylthiazole-3-carbonyl)oxy)phenyl)-4-oxo-4hydro-benzopyran-3,5,7 -Synthesis of tri-based tris(2,2-dimethylthiazole-3-carbonyl)
[0072]
[0073] Dissolve quercetin (302mg, 1.0mmol) in DMF (1ml), dilute with dichloromethane (15ml), add 2,2-dimethylthiazole-3-carbonyl chloride crude product (1.8g ), triethylamine (707mg, 7.0mmol) and DMAP (122mg, 1.0mmol). The reaction mixture was stirred overnight at 30 °C. After the reaction, water (20ml) was added, extracted with dichloromethane (20ml×3), and the combined organic phases were washed with saturated brine (20ml). Then distilled under reduced pressure to obtain a yellow oil, the crude product was separated and purified by silica gel column chromatography (eluent: dichloromethane:methanol=100:1) to obtain a white solid (175mg, 17.2%): 1 H NMR (400MHz, CDCl 3 )δ7.82(s, 2H), 7.58(m, 1H), 7.46(s, 1H), 7.18(s, 1H), 4.04(m, 10H), 3.00(s, 10H), 1.84(m, 30H ).MS: C45H55N5O12S5, calculated 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com