Pleuromutilin derivatives having alkaline parahelium side chains, and preparation method and application thereof
A technology for pleuromutilin and derivatives, which is applied in the field of pleuromutilin derivatives and their preparation, can solve the problems of unsuitability for systemic and oral administration, low bioavailability, reduced activity and the like, and achieves good in vitro antibacterial properties. Active, easy to popularize and apply, simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
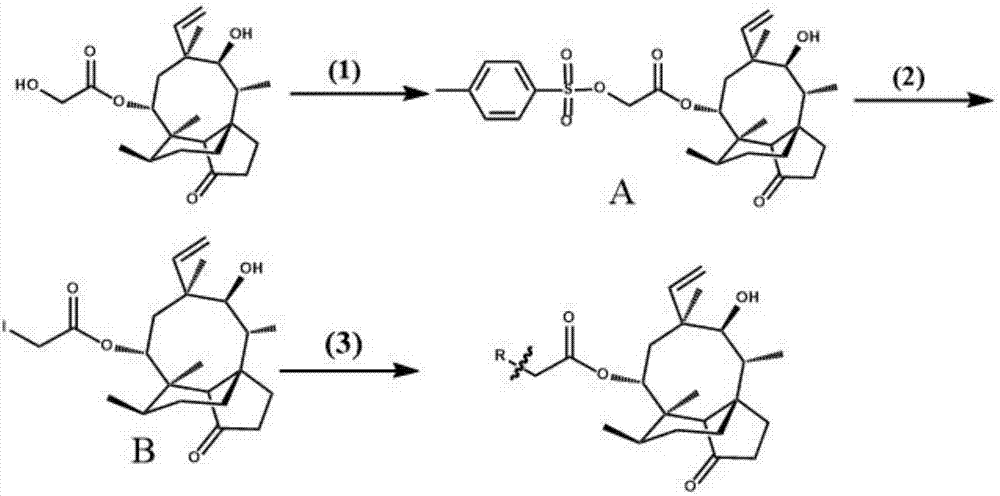
[0059] The preparation of embodiment 1 intermediate A
[0060] Take 5.4g (14.27mmol) of pleuromutilin and dissolve it in 30ml of pyridine, cool it in ice to about 0°C, and add 8.6g (45.11mmol) of p-toluenesulfonyl chloride. The reaction was stirred in an ice bath for 3 h, and then 50 ml of ice water was added to quench the reaction. Pour the reaction solution into a separatory funnel, first add 50ml of chloroform to separate layers, remove the water phase, then wash the organic phase twice with 100ml of sulfuric acid solution with a concentration of 2mol / L, and then wash the organic phase twice with 50ml of saturated sodium bicarbonate solution , and finally the organic phase was washed twice with 100 ml of deionized water and dried with anhydrous sodium sulfate. Dry the organic phase by rotary evaporation, add 10ml of isopropanol to the residual solid and heat to dissolve. After cooling, a large amount of white powder is precipitated, filtered under reduced pressure, and the...
Embodiment 2
[0061] The preparation of embodiment 2 intermediate B
[0062] Dissolve 1 g (1.88 mmol) of intermediate A obtained in Example 1 in 35 ml of ethyl acetate, add 0.31 g (2.07 mmol) of anhydrous sodium iodide, and heat and stir at 70°C for 1 h. Pour the reaction solution into a separatory funnel, add 50ml of 15% brine, and then extract with 30ml of chloroform, take the organic phase, repeat three times, dry over anhydrous sodium sulfate, filter the obtained organic phase and rotate to dryness to obtain the mixture after the subsequent reaction solvent Redissolved to obtain intermediate B, such as figure 1 As shown, the yield was 90%.
Embodiment 3
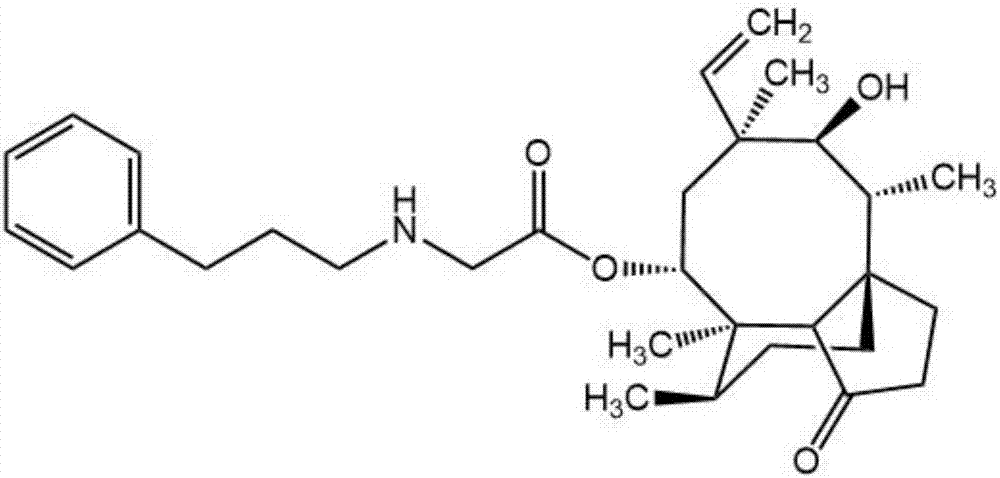
[0063] Example 3 Synthesis of 14-O-(phenylpropyl)aminoacetyl muulin (compound 1)
[0064] Dissolve intermediate B in 5ml of acetonitrile, add 5ml of acetonitrile solution containing amphetamine (2.07mmol) dropwise, then add potassium carbonate powder (4.14mmol), heat and stir at 75±1°C for 4h, pour the reaction solution into a separatory funnel, add 50ml of 15% brine and 30ml of chloroform were added for extraction, and the organic phase was taken, repeated three times, dried over anhydrous sodium sulfate, and filtered to obtain a mixture. The obtained mixed solution was rotary evaporated to dryness, and the mixture was redissolved in dichloromethane, and 1 g of 100-200 mesh silica gel was added to mix thoroughly. After the solvent evaporated, the above-mentioned crude product-silica gel powder mixture was purified by column chromatography (200-300 Mesh silica gel powder is the stationary phase, petroleum ether: ethyl acetate=2:1 is mobile phase), obtains the pure product of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com