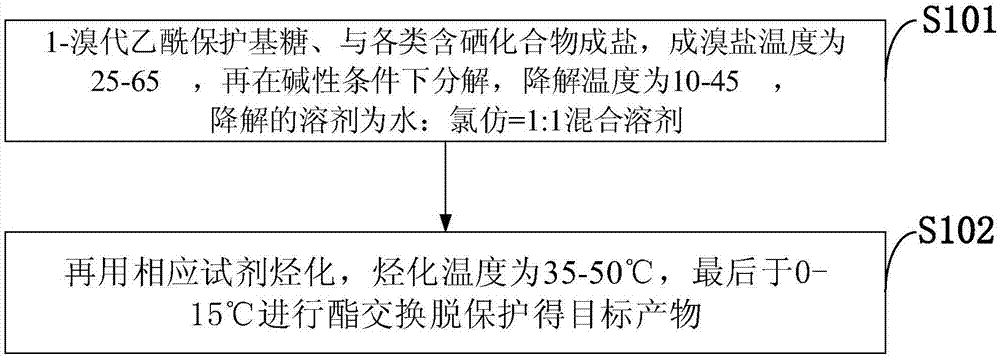
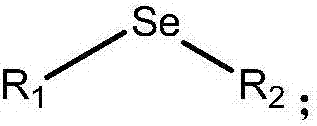
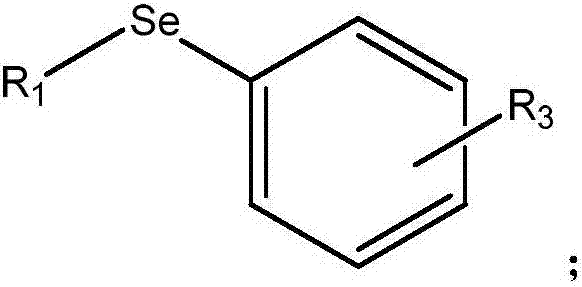
Selenium-containing compound selenium sugar, selenium glucoside and preparation method thereof
A technology for selenium compounds and glucose, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of complex components, difficult to obtain raw materials, and difficult separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] like figure 1 Shown, the preparation method of the selenium-containing compound that the embodiment of the present invention provides comprises the following steps:
[0060] S101: 1-bromoacetyl protecting group sugar, forms salt with various selenium-containing compounds, the temperature of forming bromine salt is 25-65°C, and then decomposes under alkaline conditions, the degradation temperature is 10-45°C, and the degradation solvent is Water: chloroform = 1:1 mixed solvent;
[0061] S102: Then use corresponding reagents to alkylate, the alkylation temperature is 35-50°C, and finally carry out transesterification and deprotection at 0-15°C to obtain the target product.
[0062] The preparation method of the selenium-containing compound that the embodiment of the present invention provides comprises:
[0063] i) reacting the corresponding bromosugar protected with a protecting group with selenourea, and then degrading it under alkaline conditions, or
[0064] ii) reac...
Embodiment 1
[0072] Example 1 Preparation of 2,3,4,6-O-tetraacetyl-α-D-glucose-1-isoselenuronium hydrobromide
[0073] Add 30ml of acetone into a 50ml three-necked flask, add 2.5g of selenourea and reflux for 30 minutes, cool down to 30°C, 10g of 2,3,4,6-O-tetraacetyl-α-D-1-bromoglucose Add the system, keep the temperature between 30-35°C, and react for 2 hours. Cool down to 0°C and stir for 40 minutes, filter, recrystallize the filter cake with acetone, filter, and dry to obtain an off-white solid. 1H NMR (DMSO-d6) δ2.02(8.91H); δ2.05(2.99H); δ3.79-3.83(t, 1.05H); δ4.07-4.11(t, 0.97H); δ4.82 -4.88(m, 2.03H); δ5.03-5.04(d, 0.99H); δ5.28-5.31(t, 1.01H); δ5.39-5.42(t, 1.01H); δ6.99(2.98 h)
Embodiment 2
[0074] Example 2 Preparation of 1-seleno-2,3,4,6-O-tetraacetyl-α-D-glucose
[0075] Add 10 grams of 2,3,4,6-O-tetraacetyl-α-D-glucose-1-isoselenuronium hydrobromide into 30ml of water and 30ml of chloroform, stir for 30 minutes, add 10 grams of sodium bicarbonate , stirred at room temperature for 5 hours. The liquid was separated, the organic layer was washed with water, dried and concentrated, and the oil was crystallized from ether to obtain a yellow-green waxy solid. 1H NMR (DMSO-d6) δ1.98-2.02(d, 11.96H); δ4.15-4.21(m, 2.01H); δ4.41-4.44(q, 1.02H); δ4.47-4.49(m , 0.99H); δ5.25-5.27(t, 1.00H); δ5.39-5.41(t, 1.00H); δ5.94-5.96(t, 1.00H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com