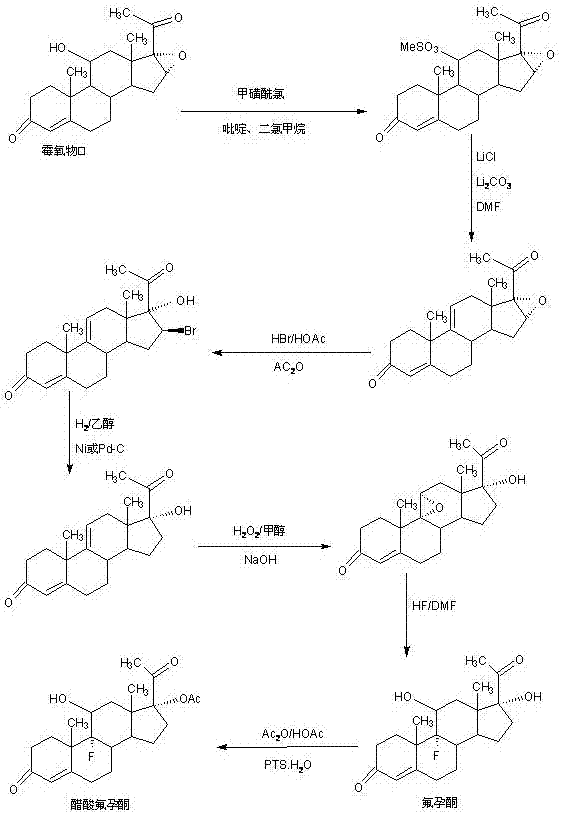
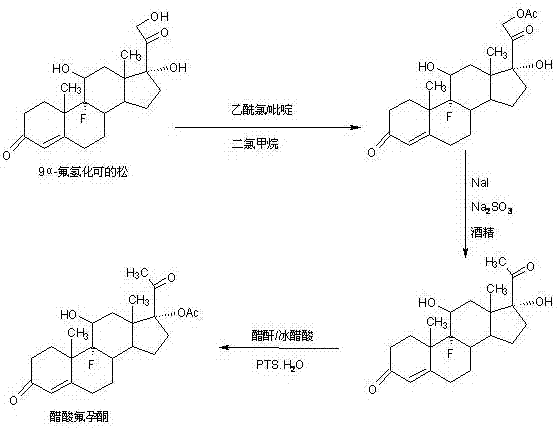
Preparation method of flurogestone acetate
A technology for fluprogesterone acetate and fluprogesterone acetate is applied in the field of preparation of three-step chemical reaction to synthesize fluprogesterone acetate, which can solve the problems of complex process operation, long synthesis route and high production cost, and achieves wide raw material sources and synthetic route. Short, low production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A. Synthetic esters
[0019] In a 2000ml three-necked bottle, add 100g 9a-fluorocortisone, 600ml toluene, stir at room temperature to dissolve, then add 80g triethylamine, cool down to 10-15°C, slowly add dropwise 40g methanesulfonyl chloride and Add the solution made of 200ml toluene within about 0.5-1.0 hours, and then keep it warm at 20-25°C for 3-4 hours. TLC confirms the reaction end point. After the reaction, add 100g of 20% caustic soda aqueous solution, Continue to stir at 20-25°C for 1-2 hours to completely destroy the acid chloride, then separate the water layer, wash it twice with 800ml of tap water, dry it with 50g of anhydrous magnesium sulfate, recover toluene under reduced pressure until it is nearly dry, and then add 10 % ethanol aqueous solution 800ml, stirred and crystallized at 5-10°C for 4-6 hours, filtered, washed, and dried to obtain esterified product: 118g of 21-O-methylsulfonyl-9a-fluorocortisone, HPLC content 98.5%, weight The yield is 118%; ...
Embodiment 2
[0025] A. Synthetic esters
[0026]In a 2000ml three-necked flask, add 100g 9a-fluorocortisone and 600ml dichloromethane, stir at room temperature to dissolve, then add 80g triethylamine, raise the temperature to 40-45°C, and slowly add 40g p-toluene dropwise Add the solution of sulfonyl chloride and 200ml toluene within 0.5-1.0 hours, and then keep it at 40-45°C for 3-4 hours, then confirm the reaction end point by TLC. After the reaction, add 100g of 20% caustic soda aqueous solution , continue stirring at 40-45°C for 1-2 hours to completely destroy the acid chloride, then cool to 20-25°C, separate the water layer, then wash with 800ml of tap water twice, dry with 50g of anhydrous magnesium sulfate, and reduce pressure Recover toluene until nearly dry, then add 800ml of 10% ethanol aqueous solution, stir and crystallize at 5-10°C for 4-6 hours, filter, wash, and dry to obtain esterified product: 21-O-methylsulfonyl-9a-fluorocortisone 138g, HPLC content 98.3%, weight yield i...
Embodiment 3
[0032] A. Synthetic esters
[0033] In a 2000ml three-neck flask, add 100g 9a-fluorocortisone, 400ml glacial acetic acid, stir at room temperature to dissolve, then add 8g p-toluenesulfonic acid, 120g acetic anhydride, heat up to 40-45°C and keep warm for reaction After 5-6 hours, TLC confirms the end point of the reaction. After the reaction, cool to 20-25°C, add 1000g of 2% sodium carbonate aqueous solution, and continue to stir and crystallize for 3-4 hours, then filter, and the filtrate is discharged into waste water after recovering acetic acid In the treatment tank, add 800ml of 10% ethanol aqueous solution to the filter cake, stir and crystallize at 5-10°C for 4-6 hours, filter, wash, and dry to obtain esterified product: 21-O-methylsulfonyl-9a-hydrocortisone 108g , HPLC content 98.5%, weight yield is 108%;
[0034] B. Synthetic fluprogesterone
[0035] In a 1000ml three-neck flask, add 100g of the esterified product obtained above, 600ml of alcohol, stir at room temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com