Application of imine reductase and mutant thereof in synthesis of (S)-1-aryl-1, 2, 3, 4-tetrahydroisoquinoline
A reductase and mutant technology, applied in the field of enzyme engineering, can solve the problems of unavailable catalysts, large steric hindrance of phenyl substituents, complex and harsh operating conditions, etc., achieve high product conversion rate and optical purity, and simplify post-processing steps , the effect of improving the quality of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 Obtaining of imine reductase of the present invention
[0046] (1) Acquisition of the gene encoding imine reductase
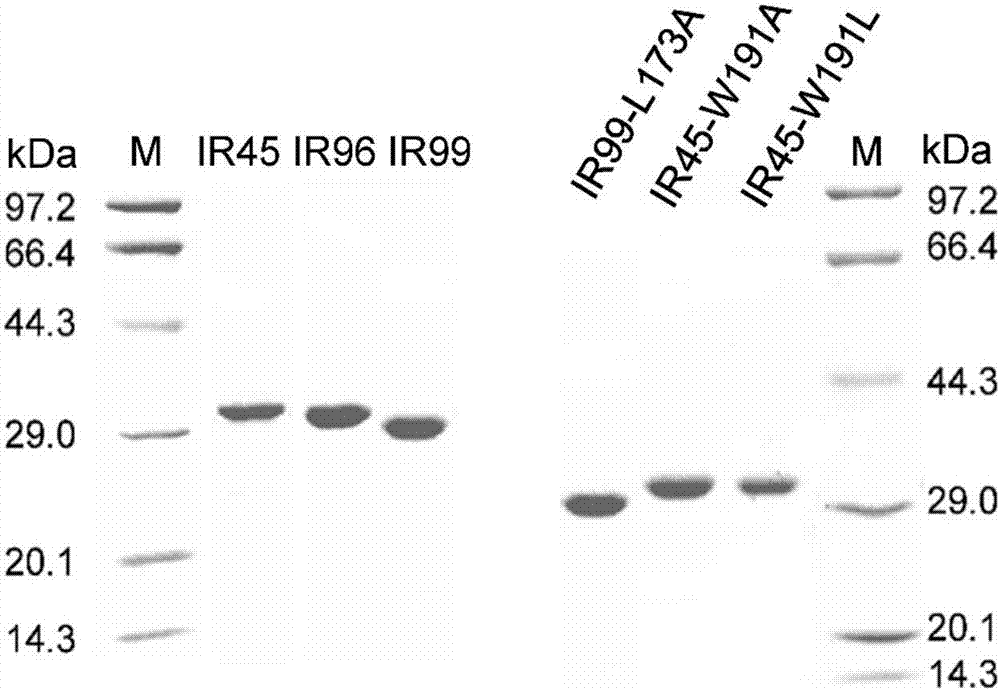
[0047] The three imine reductases (IR45, IR96 and IR99) with catalytic ability obtained in the present invention are obtained by screening the imine reductase enzyme library to catalyze the reaction of compounds 1a-4a (the following formula). The imine reductase enzyme library is the 100 genes that may encode imine reductase constructed by the applicant in the early stage. The DNA sequences of these 100 genes are optimized according to the codon preference of Escherichia coli and sent to the company for synthesis. The codon-optimized genes were respectively added with NdeI (CATATG) restriction site at the 5' end and HindⅢ (TTCACT) restriction site at the 3' end, and cloned into pET28a plasmid to obtain recombinant plasmids pET28a-IR45, pET28a- IR96, pET28a-IR99.
[0048]
[0049] The amino acid sequences of imine reductases IR45, IR97 a...
Embodiment 2
[0056] The synthesis of embodiment 2 imine substrates
[0057] (1) Synthesis of 1-m-methylphenyl-3,4-dihydroisoquinoline
[0058] The synthetic route is as follows:
[0059]
[0060] Under the protection of nitrogen, add 2.5mL of phenethylamine (20mmol, 1.0equiv) and 60mL of anhydrous dichloromethane into the previously dried 250mL round bottom flask, cool down to 0°C, and slowly add 4.2mL of triethylamine (30mmol , 1.5equiv) and 2.9mL (22mmol, 1.1equiv) of 3-methylbenzoyl chloride, reacted at 0°C for 30 minutes after the dropwise addition, and then rose to room temperature for 24 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and 50mL was added 1M HCl, extracted three times with ethyl acetate, 50 mL each time, combined the ethyl acetate phases, washed once with water, washed once with saturated brine and dried with anhydrous magnesium sulfate for 1 hour, and the solvent was removed by rotary evaporation to obtain the crude product ...
Embodiment 3
[0072] Synthesis of embodiment 3 imine reduction product racemate
[0073] (1) Synthesis of 1-phenyl-1,2,3,4-tetrahydroisoquinoline
[0074] Synthesized by the following route:
[0075]
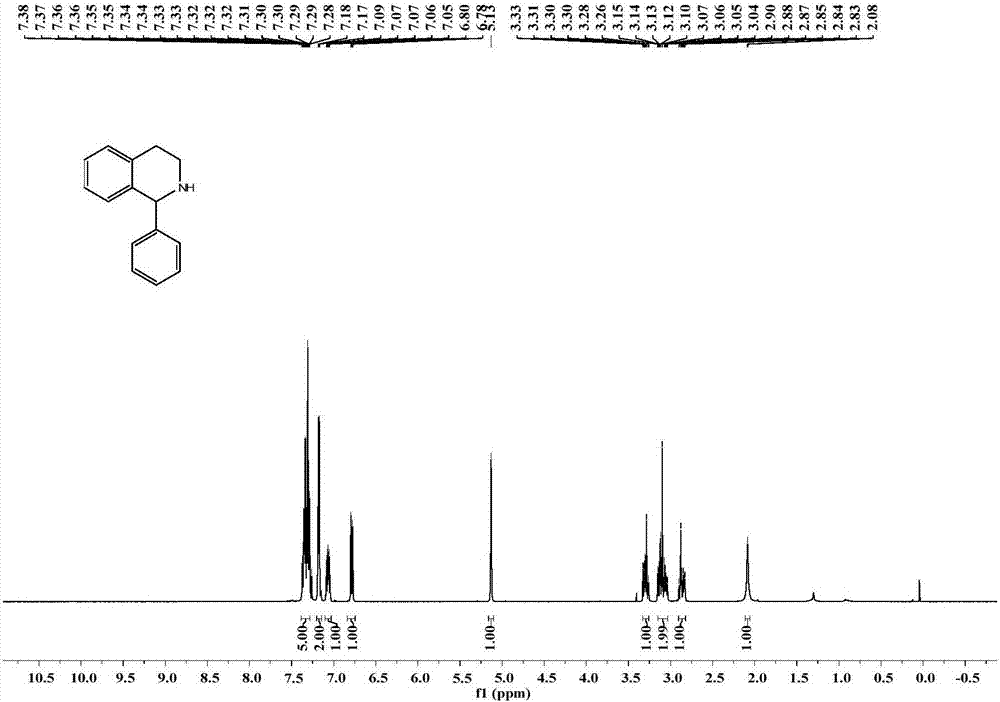
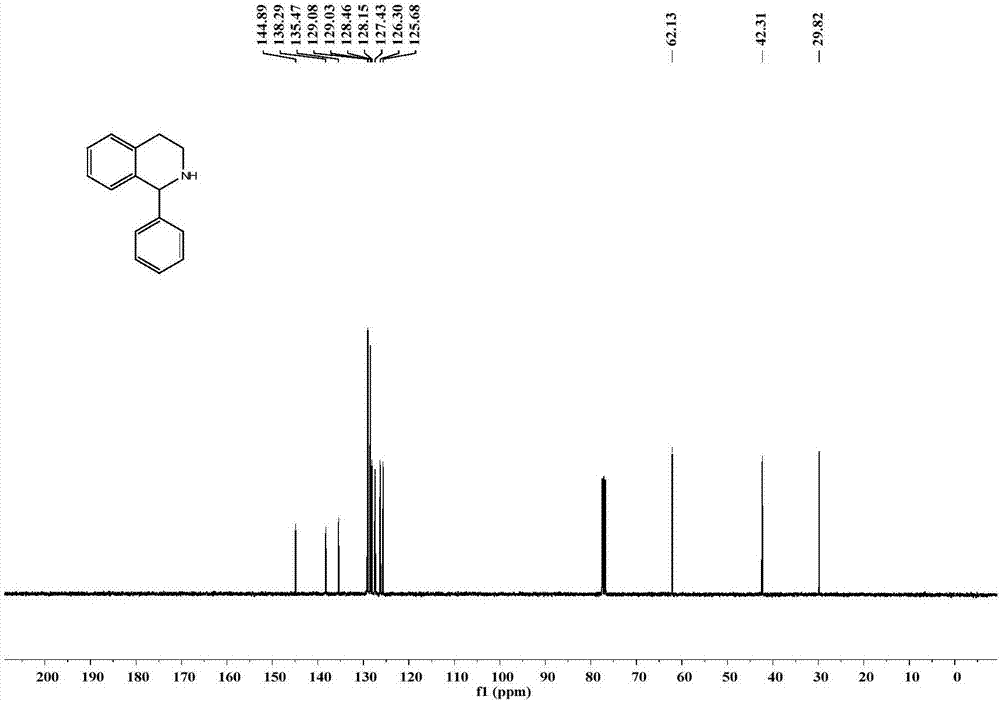
[0076] The specific operation is as follows: under the condition of nitrogen protection, in a 10 mL pre-dried round bottom flask, add 0.5 mmol 1-phenyl-3,4-dihydroisoquinoline (1a), add 5 mL of anhydrous methanol, and cool down to 0°C, add 0.6mmol NaBH 4 , after stirring at 0°C for 30 minutes, warm to room temperature and stir for 2 hours, add 20 mL of water to quench the reaction, extract three times with ethyl acetate, 20 mL each time, combine the ethyl acetate phases and dry with anhydrous magnesium sulfate for 30 minutes, spin dry to remove The solvent racemate 1-phenyl-1,2,3,4-tetrahydroisoquinoline (rac-1b) was a white solid with a yield of 96%.
[0077] Compound rac-1b NMR / MS data ( 1 H and 13 C spectrum see figure 2 , 3 ): 1 H NMR (400MHz, Chloroform-d) δ7.38–7.28(m,5H),7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com