Ursolic acid neutral cholesterol ester hydrolase inhibitor and application thereof
A cholesterol lipid and inhibitor technology, applied in the field of biomedicine, can solve the problems of reducing the oral bioavailability of drugs, achieve high inhibitory activity, simple synthesis process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of 3-carboxypropionyl-ursolic acid
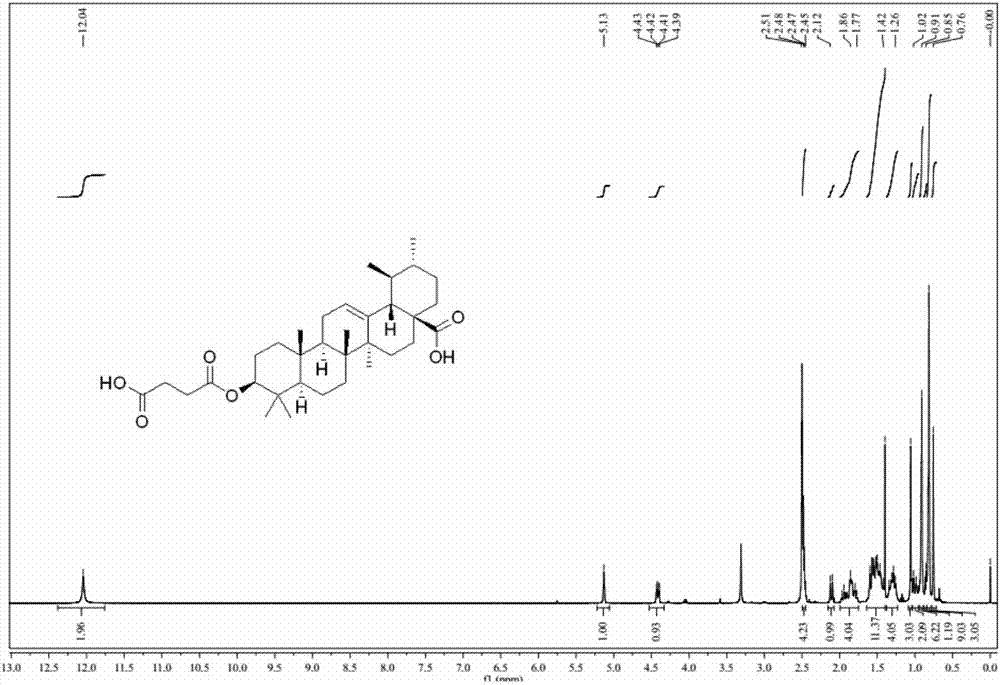
[0026] At room temperature, successively add ursolic acid (228.4mg, 0.5mmol), 4-dimethylaminopyridine (122.4mg, 1.0mmol), succinic anhydride (200.2mg, 2mmol) into the dichloromethane (15mL) solution, and add The reaction was stirred at room temperature and the progress of the reaction was monitored. After the reaction is complete, add water (25mL), adjust the pH to 2-3 with 1M HCl solution, extract three times with ethyl acetate (40mL×3), wash the combined organic phase with water (30mL×1), wash with saturated sodium chloride solution (25mL×1 ), dried over anhydrous sodium sulfate, evaporated to remove the solvent, and the crude product was obtained by column chromatography (dichloromethane / methanol=100 / 1-20 / 1 gradient elution) to obtain a white solid with a yield of 60-70%. 1 H-NMR spectrum as figure 1 shown.
[0027] The nuclear magnetic resonance spectrum of product is specifically as follows:
[0028] 1 H NMR (400M...
Embodiment 2
[0031] Synthesis of 3-carbonyl-ursolic acid
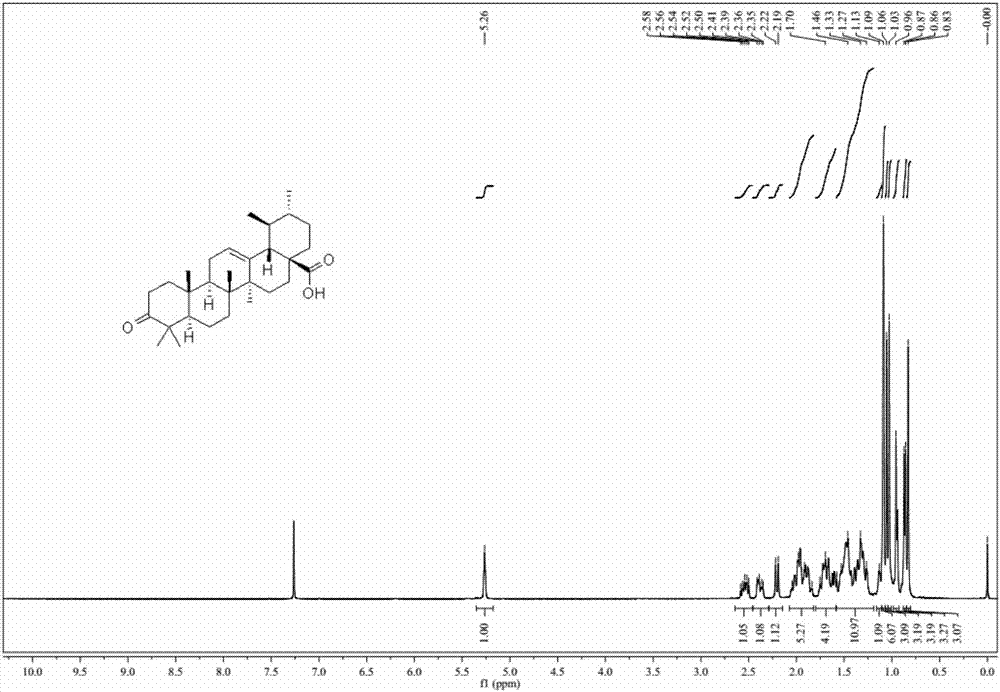
[0032] At room temperature, dissolve ursolic acid (456.7mg, 1.0mmol) in acetone (20mL) solution, cool to 0°C, and slowly add the prepared concentrated sulfuric acid solution of chromium trioxide dropwise (chromium trioxide: concentrated sulfuric acid: Water = 1.824g: 1.57mL: 7.6mL), after the addition was completed, the reaction was stirred at 0°C, and the reaction progress was monitored. After the reaction was complete, the reaction was quenched with isopropanol. After acetone was aliquoted, water (30mL) was added to dichloromethane to extract three times (50mL×3), and the combined organic phase was washed with water (30mL×1) and saturated sodium chloride solution (30mL×1 ), dried over anhydrous sodium sulfate, and evaporated to remove the solvent. The crude product was subjected to column chromatography (petroleum ether / ethyl acetate=20 / 1-5 / 1 gradient elution) to obtain a white solid with a yield of 65-75%. 1 H-NMR spectrum as ...
Embodiment 3
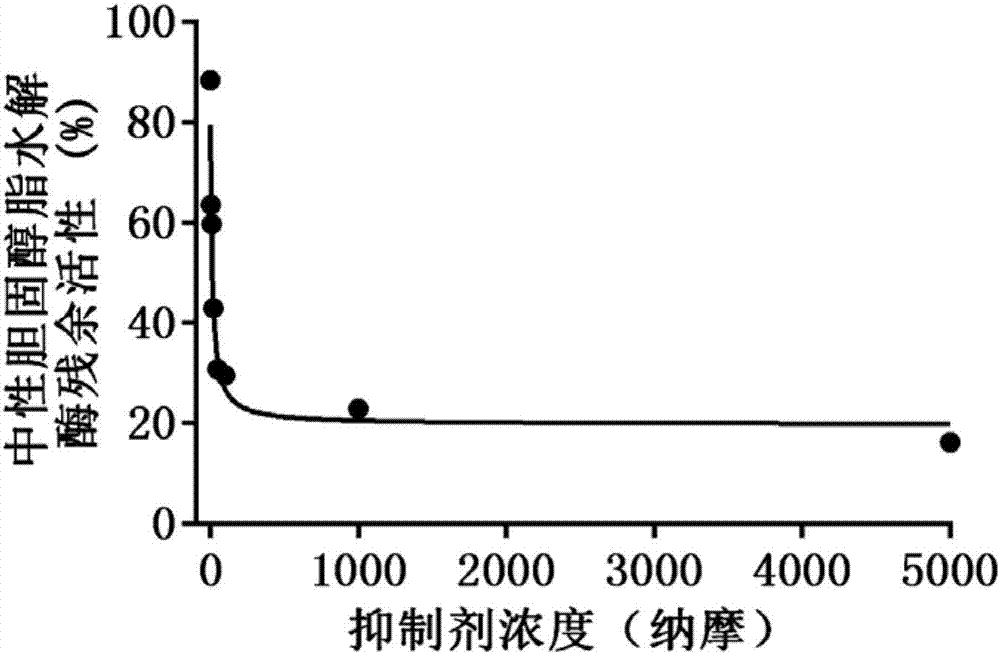
[0037] Quantitative evaluation of the inhibitory ability of ursolic acid compounds on neutral cholesterol lipohydrolase (NCEH1)
[0038] Using the hydrolytic metabolism of D-fluorescein methyl ester (DME) as a probe reaction, bioluminescence was used to determine the inhibitory IC of ursolic acid compounds on neutral cholesterol lipohydrolase (NCEH1) with the help of human liver microsome incubation system in vitro 50 :
[0039] a. In 50 microliters of in vitro metabolic reaction system, containing phosphate buffer solution with a pH of 6.5, the concentration of human liver microsomal protein is 2 μg / ml, and the final concentration of inhibitors is in the range of 0.1 μM-80 μM. Pre-incubation with shaking on the instrument for 10 minutes;
[0040] b. Add D-fluorescein methyl ester (DME) substrate (final concentration 3 μM) to the reaction system to initiate the reaction, and incubate the reaction with shaking on a microplate reader for 10 minutes;
[0041] c. Add Luciferin D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com