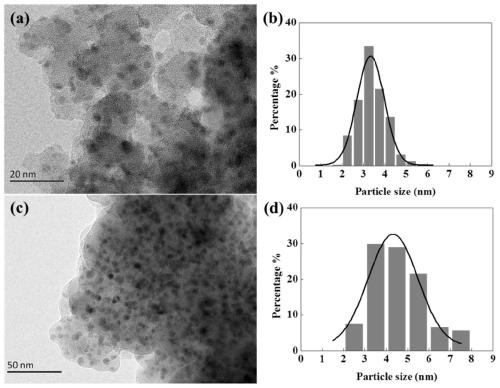
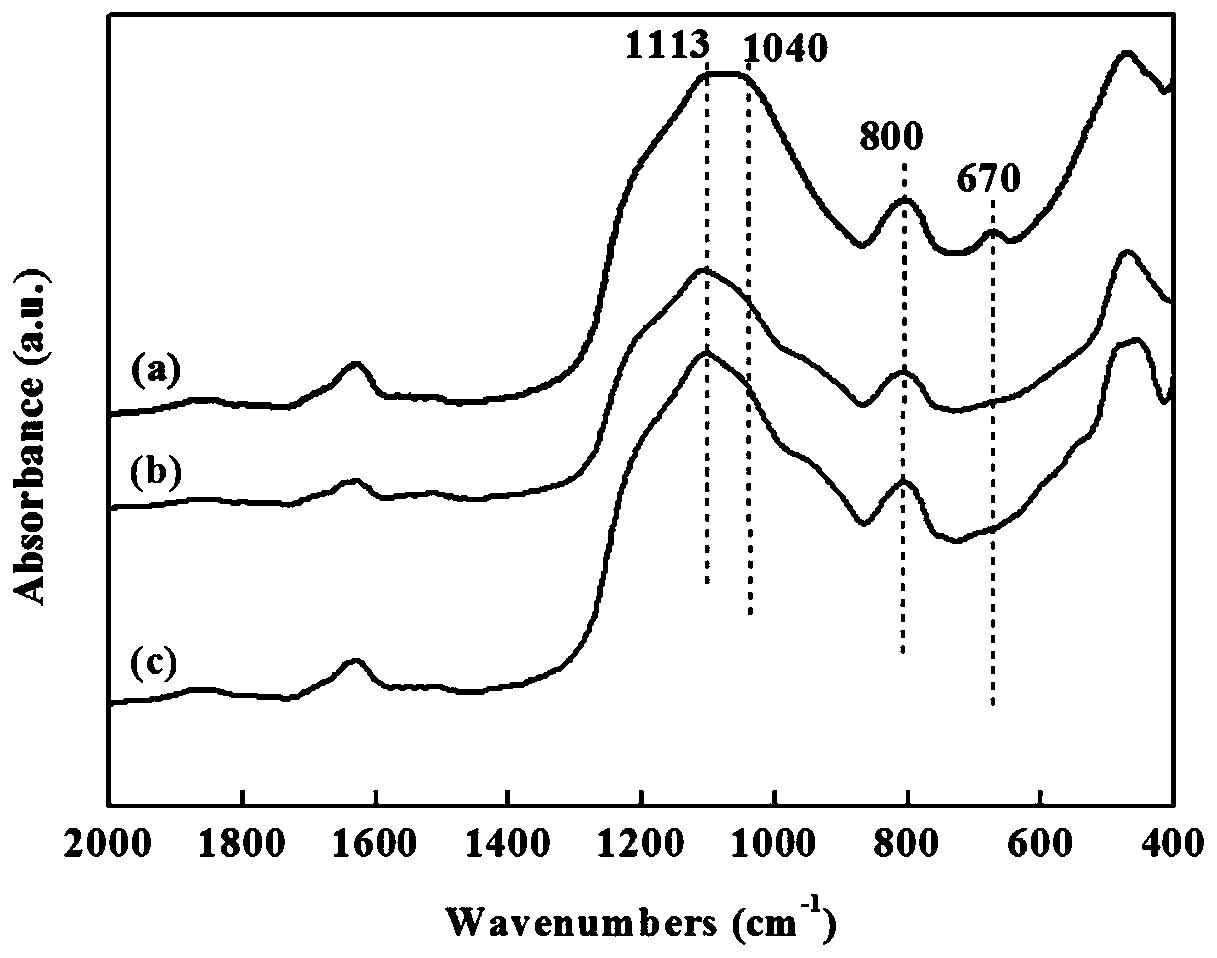
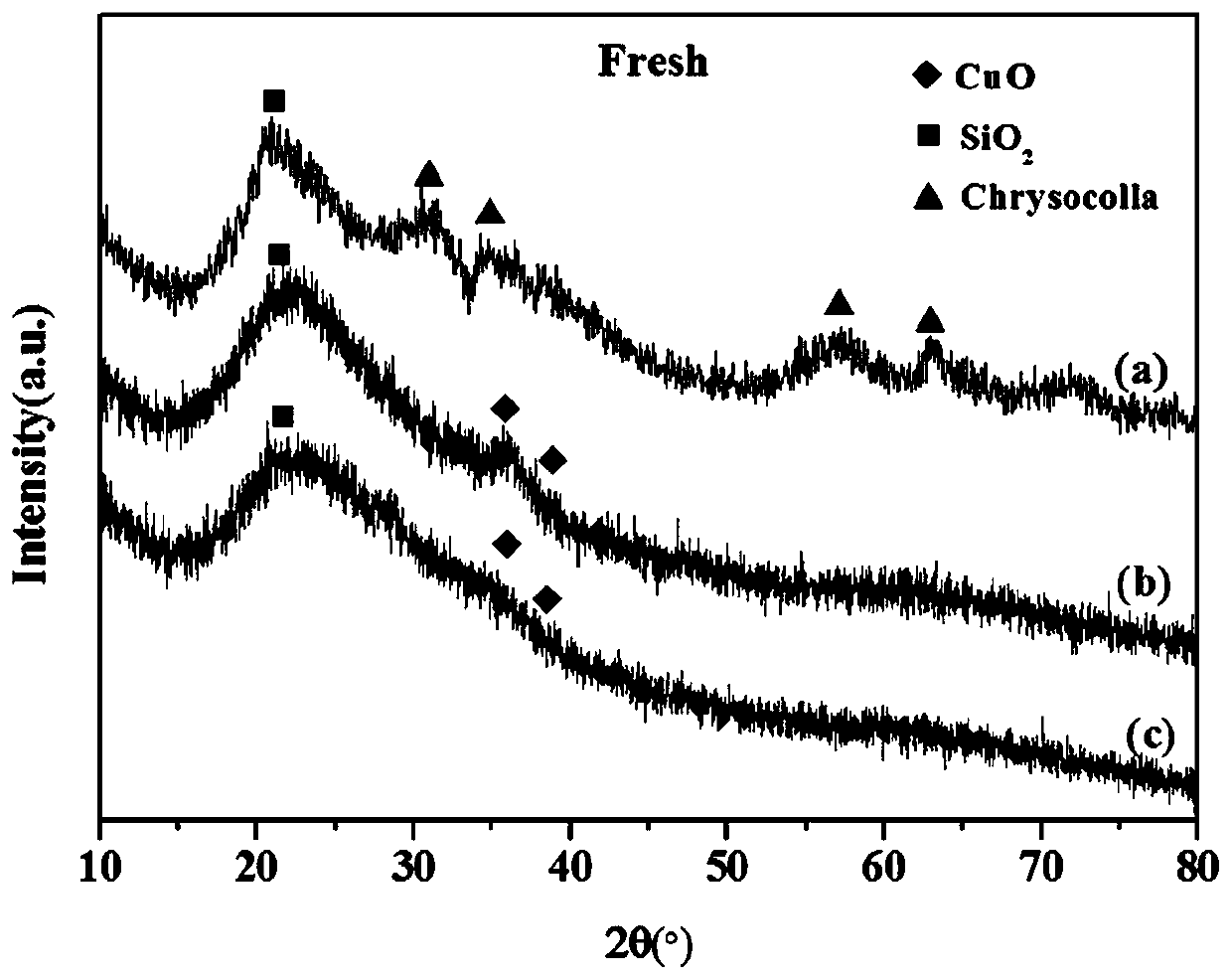
Copper-based hydrogenation catalyst prepared by flame jet cracking method and its preparation and application
A technology of hydrogenation catalyst and flame injection, which is applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, heterogeneous catalyst chemical elements, etc. Environmental and human factors have a large impact, hindering the scale-up production of catalysts, etc., to achieve the effect of avoiding sintering aggregation, small environmental and human factors, and obvious modification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1.89g copper acetate Cu(CH 3 COO) 2 ·H 2 O and 11.2ml of tetraethyl orthosilicate (TEOS) were dissolved in a mixed solvent of 44.4ml of methanol and 44.4ml of 2-ethylhexanoic acid. The solution was placed on a magnetic stirrer and stirred at room temperature until a clear solution was obtained. Use a syringe to pump the prepared solution into the nozzle at a rate of 5 ml / min. Flame combustion gas is a mixture of methane (0.6L / min) and oxygen (1.9L / min), dispersion gas is oxygen (3.5L / min, pressure drop 4.5bar), protective gas is air (5.0L / min) . Catalyst particles from combustion were collected with glass fiber filter paper with the help of a vacuum pump. The prepared catalyst is denoted as FSP-Cu / SiO 2 , the mass fraction of Cu is 20%.
[0035] Reduction conditions before catalyst reaction: under normal pressure, pure H 2 (25ml / min), the temperature is 350°C, and the reduction time is 3h. Reaction conditions: molar ratio H 2 / Methyl acetate (MA) = 80, the te...
Embodiment 2
[0037] 1.89g copper acetate Cu(CH 3 COO) 2 ·H 2 O, 0.80g magnesium acetate Mg(CH 3 COO) 2 4H 2 O and 11.2ml of tetraethyl orthosilicate (TEOS) were dissolved in a mixed solvent of 44.4ml of methanol and 44.4ml of 2-ethylhexanoic acid. The solution was placed on a magnetic stirrer and stirred at room temperature until a clear solution was obtained. Subsequent steps are the same as in Example 1. The prepared catalyst is denoted as FSP-Cu-Mg / SiO 2 , the mass fraction of Cu is 20%, and the mass fraction of MgO is 5%. The reduction conditions and reaction conditions of the catalyst are the same as in Example 1. The test results (see Table 2) show that as the reaction temperature increases, the methyl acetate conversion rate increases gradually, and the ethanol selectivity increases gradually. At 200°C, the conversion rate of methyl acetate was 15.55%, which was 7.5% higher than that of the catalyst without additives (Example 1). It can be seen that in the preparation of c...
Embodiment 3
[0039] 1.89g copper acetate Cu(CH 3 COO) 2 ·H 2 O, 0.41g zinc acetate Zn(CH 3 COO) 2 2H 2 O and 11.2ml tetraethyl orthosilicate (TEOS) were dissolved in a mixed solvent of 44.4ml methanol and 44.4ml 2-ethylhexanoic acid, the mass fraction of Cu was 20%, and the mass fraction of ZnO was 5%. The solution was placed on a magnetic stirrer and stirred at room temperature until a clear solution was obtained. Subsequent steps are the same as in Example 1. The prepared catalyst is denoted as FSP-Cu-Zn / SiO 2 . The reduction conditions and reaction conditions of the catalyst are the same as in Example 1. The test results (see Table 3) show that as the reaction temperature increases, the conversion of methyl acetate increases gradually, and the ethanol selectivity increases gradually. At 200°C, the conversion rate of methyl acetate was 23.80%, which was 15.8% higher than that of the catalyst without additives (Example 1). It can be seen that in the preparation of copper-based c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com