Cyclic diketopyrrolopyrrole conjugated compounds as well as preparation method and application thereof
A technology of diketopyrrolopyrrole and diketopyrrolopyrrole, which is applied in the field of diketopyrrolopyrrole cyclic conjugated compounds, can solve the problems of no diketopyrrolopyrrole and no application, and achieve mild conditions and easy operation Easy, strong absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The synthetic route of the diketopyrrolopyrrole cyclic conjugated molecule (referred to as C-DPP1) in this example is as follows:
[0058]
[0059] Concrete synthetic steps are:
[0060] (1) Into a reaction flask containing 850.4 mg of bistin-substituted diketopyrrolopyrrole 1 was added 374.2 mg of (1,5-cyclooctadiene)platinum(II) dichloride. Under nitrogen protection, inject 400ml of anhydrous tetrahydrofuran (THF), and stir at 70 degrees Celsius; after reacting for 12 hours, the product is cooled and spin-dried, and about 50ml of methanol is added, filtered, and the filter cake is placed in a vacuum drying oven at 50 degrees Celsius for 5 hours to obtain Product 2a.
[0061] (2) Add 554.4 mg of 1,1'-bis(diphenylphosphino)ferrocene (dppf) into the reaction flask containing the product 2a obtained in the above steps. Under the protection of nitrogen, inject 400ml of anhydrous dichloromethane, stir and react at room temperature for 12 hours, spin dry the product aft...
Embodiment 2
[0067] The synthesis route of the diketopyrrolopyrrole ring-conjugated molecule (referred to as C-DPP2) in this example is as follows:
[0068]
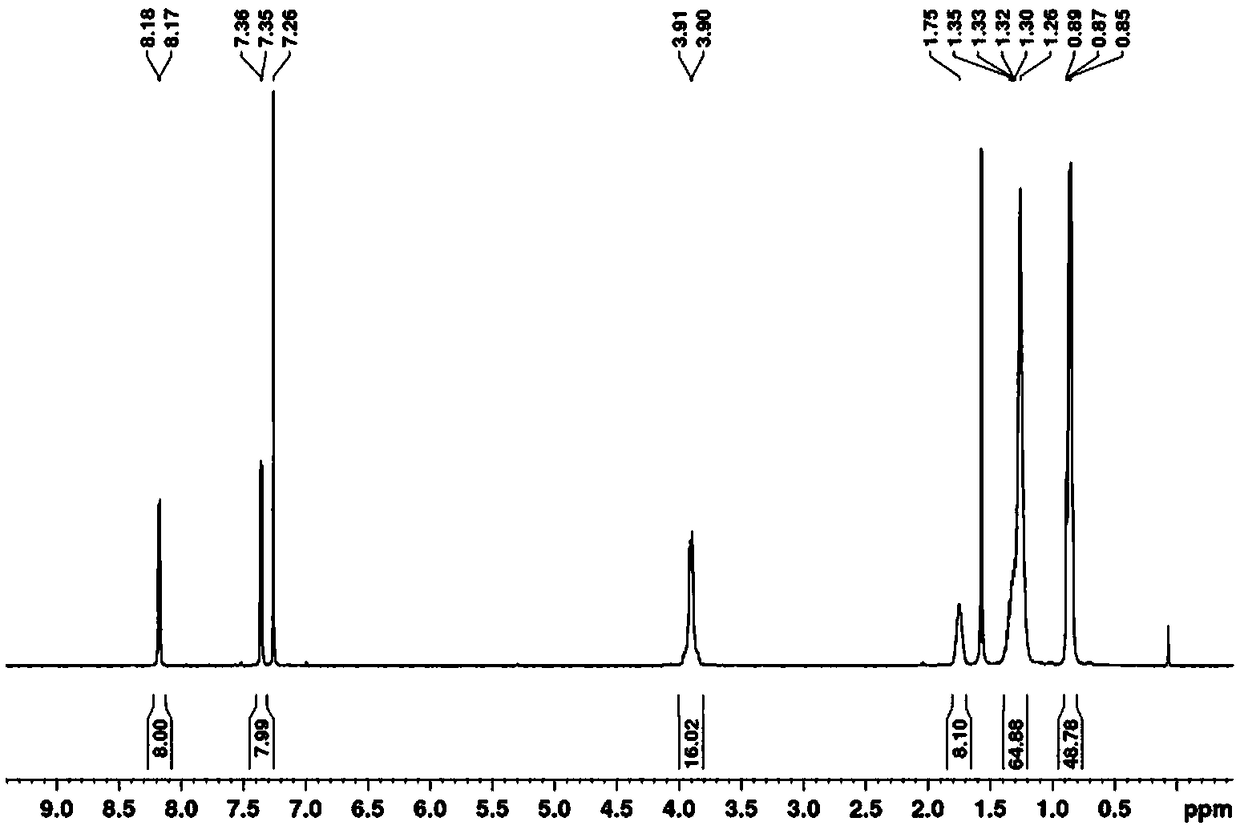
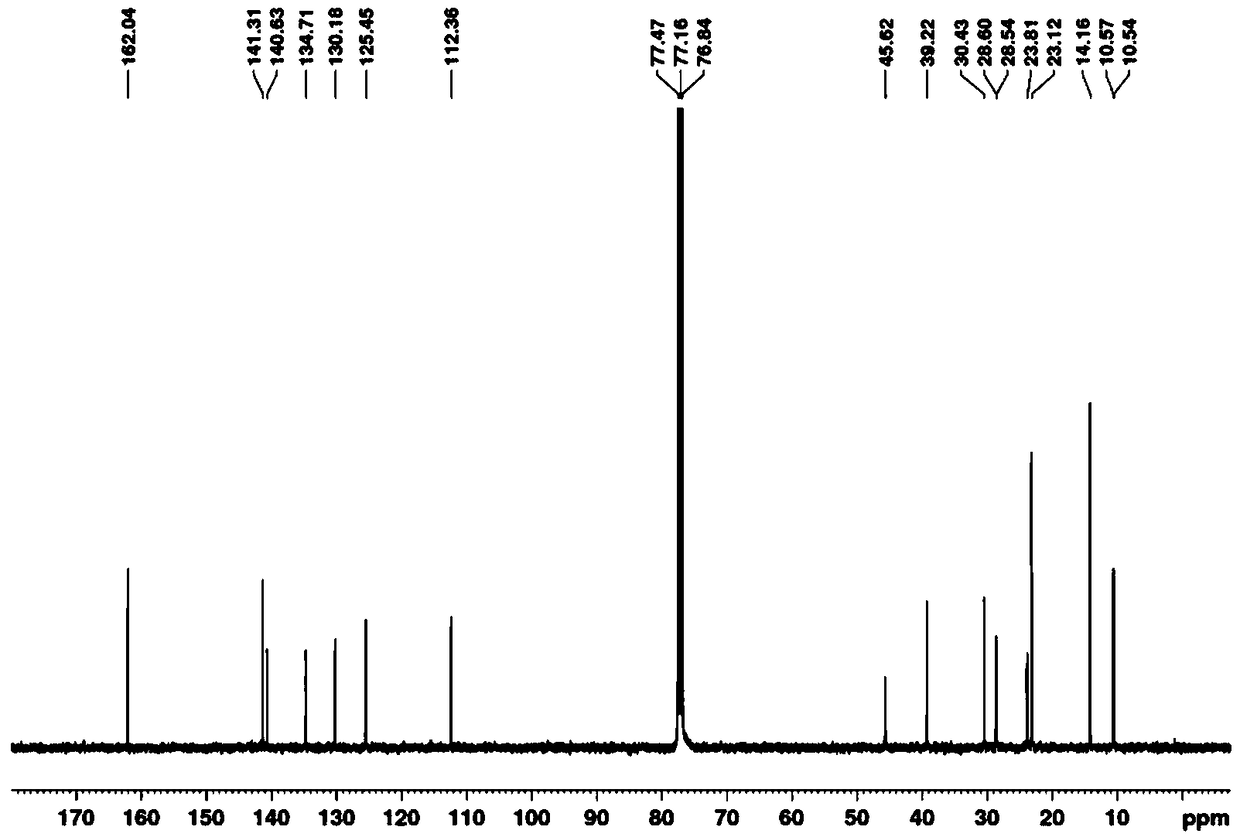
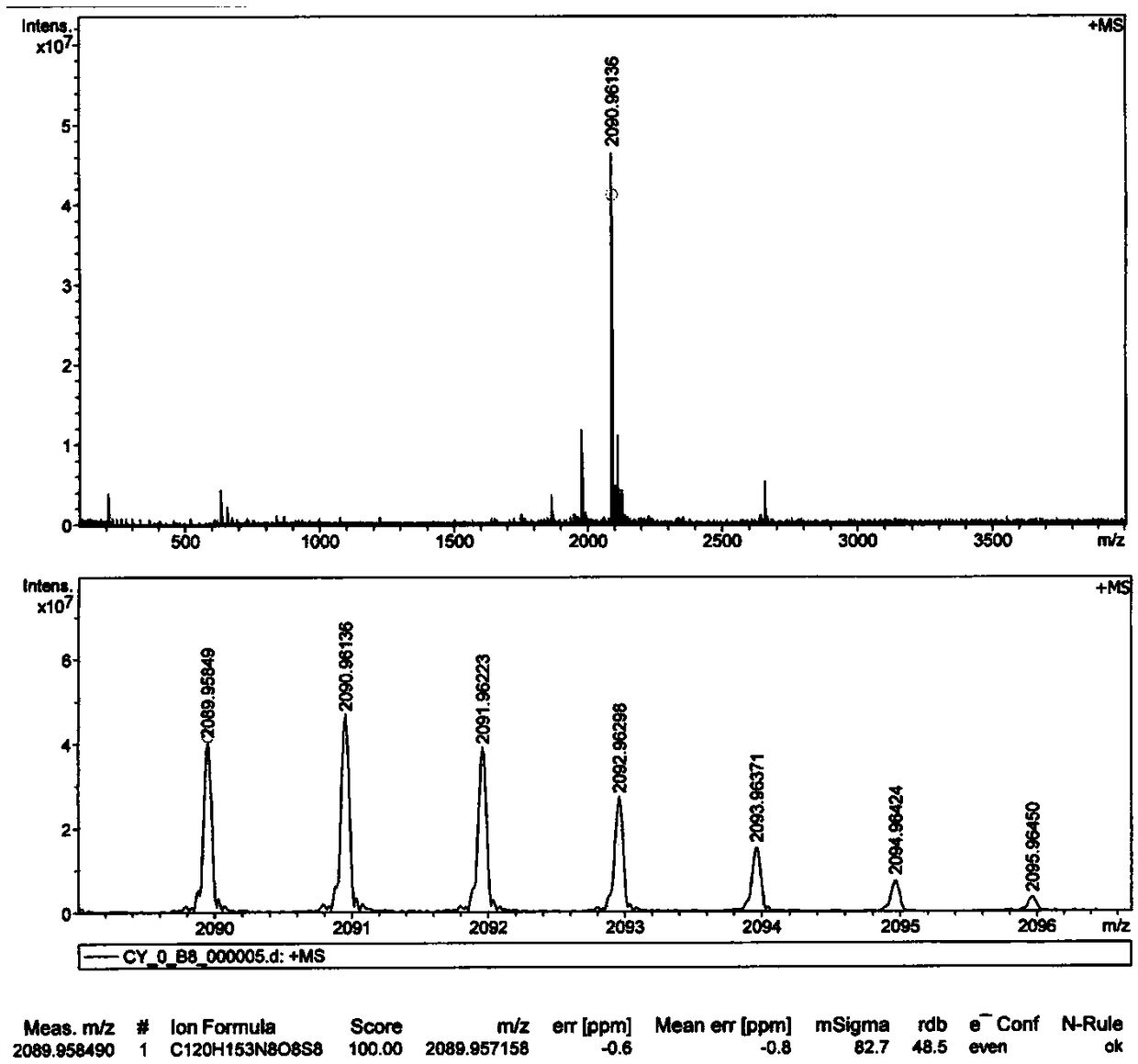
[0069] The specific synthesis steps are consistent with Example 1 of the present invention, the only difference is that the n-octyl-substituted 3 is used as the starting material, and the structure confirmation results of C-DPP2 are as follows: 1 H-NMR picture as Figure 4 as shown, 13 C-NMR picture as Figure 5 As shown, the mass spectrum is as Figure 6 shown.
Embodiment 3
[0071] Diketopyrrolopyrrole ring-conjugated molecular field-effect transistor devices:
[0072] The field effect transistor device structure is bottom gate-top contact, the specific structure is as follows Figure 7 As shown: the source and drain electrodes are all vapor-deposited gold layers with a thickness of 25nm, the gate electrode is silicon with a thickness of 400μm, the insulating layer is silicon dioxide modified with OTS (octadecyltrichlorosilane) with a thickness of 300nm, and the organic semiconductor part is C-DPP1 with a thickness of 40-60 nm.
[0073] Dissolve the diketopyrrolopyrrole cyclic conjugated molecule C-DPP1 obtained in Example 1 of the present invention as an active layer in a mixed solution of chloroform and n-hexane (volume ratio: 10:1), with a concentration of 10 mg / mL, using OTS (octadecyltrichlorosilane) modified silicon dioxide / silicon (SiO 2 / Si) substrate, as the organic semiconductor part of the organic field effect transistor device, the t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com