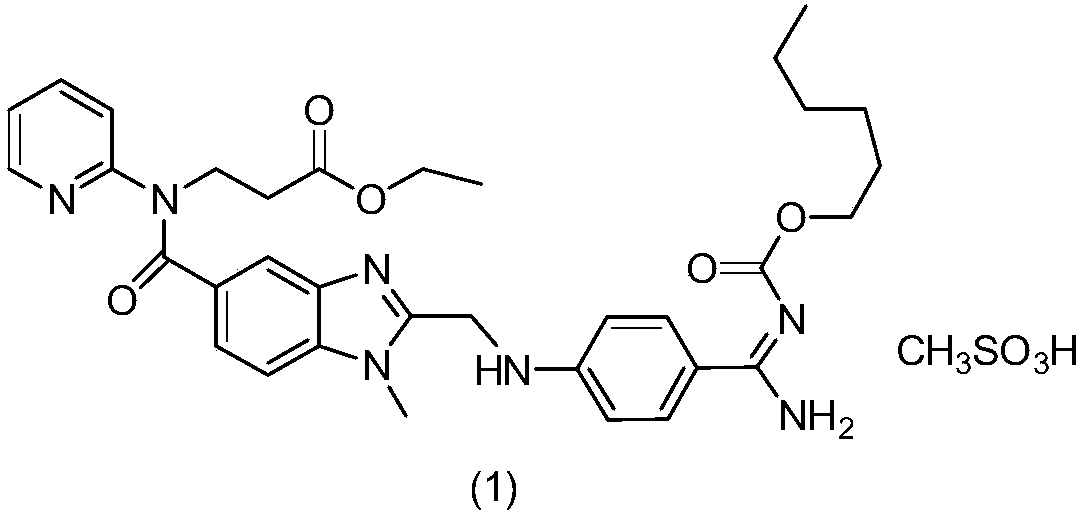
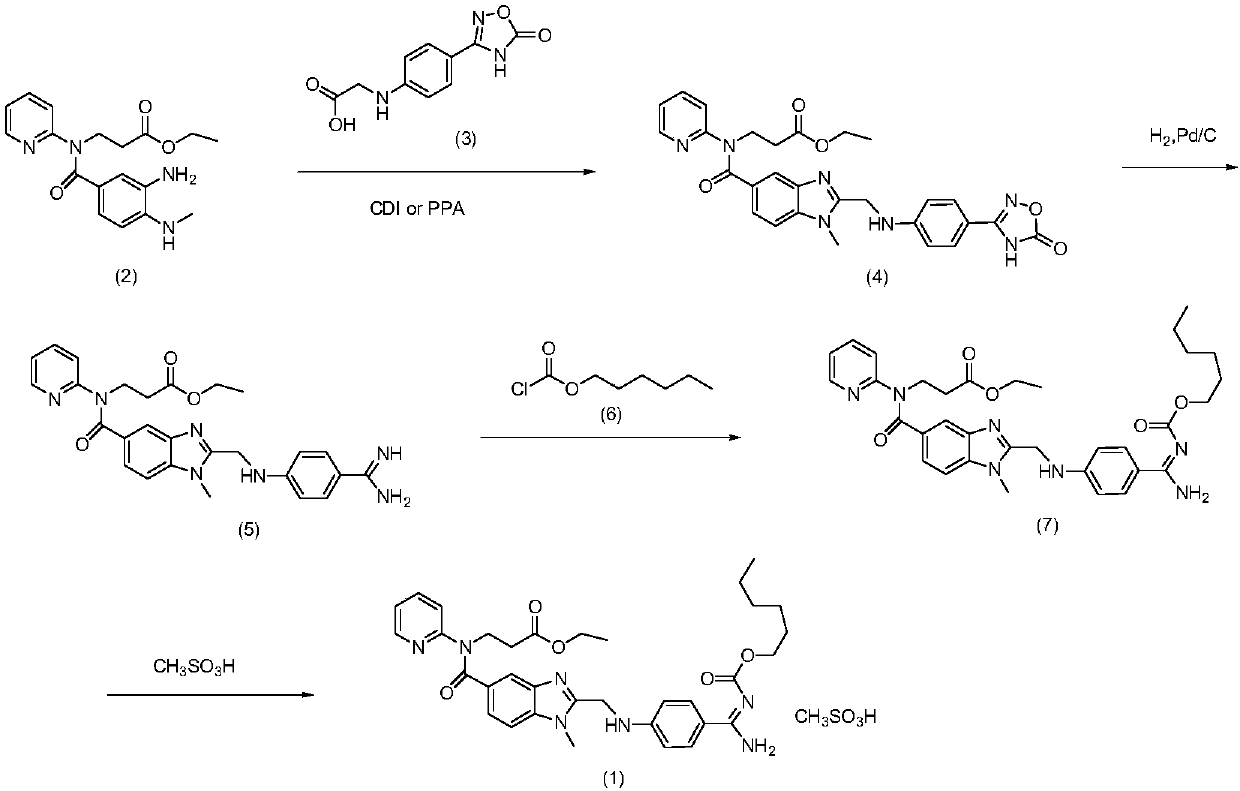
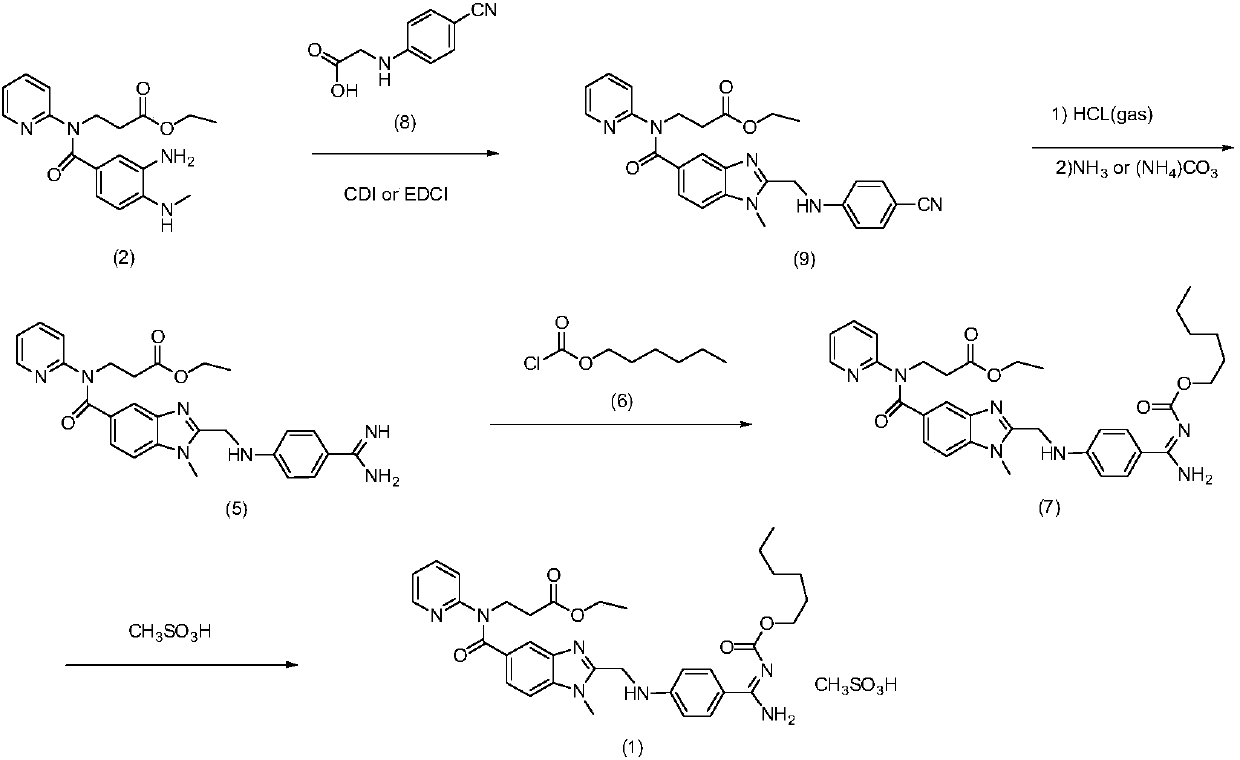
Dabigatran etexilate mesylate preparation method
A technology of dabigatran etexilate mesylate and ethyl propionate, which is applied in the field of drug synthesis, can solve the problems of many impurities, pollute the environment, and long reaction time, and achieve high purity and yield, easy-to-obtain reaction raw materials, The effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Closed loop reaction
[0039] Add 120ml of ethyl acetate, 28g of ethyl 3-[(3-amino-4-methylaminobenzoyl)(pyridin-2-yl)amino]propionate and 14.5g of chloroacetic anhydride to the reactor at room temperature. , Stir and heat to 65°C for 2 hours, then cool to 40°C, add 15g of potassium carbonate, and keep at 40°C for 4 hours. After filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was cooled to 0°C with 150ml methyl tert-butyl ether to crystallize to obtain N-[[2-(chloromethyl)-1-methyl-1H-benzimidazole-5 -Yl]carbonyl]-N-2-pyridyl-β-alanine ethyl ester 29.5 g, yield 90%, product HPLC purity 98.0%. 1 H-NMR(CDCl 3 )δ1.1(t,3H, -CH 3 ),δ2.7(t,2H,-CH 2 -),δ2.8(s,3H,-NCH 3 ),δ4.1(m,2H,-NCH 2 -),δ4.2(s,2H,-CH 2 -),δ4.4(t,2H,-CH 2 Cl),δ6.5(m,1H,Ar-H),δ6.8(m,1H,Py-H),δ7.2(m,2H,Ar-H),δ7.4(m,1H, Py-H), δ8.0 (m, 1H, Py-H), δ8.5 (m, 1H, Py-H).
[0040] (2) Condensation reaction
[0041] Add 1.5 g of sodium iodide, 6.6 g of potassium carbonate, a...
Embodiment 2
[0047] (1) Closed loop reaction
[0048] Add 120ml of ethyl acetate, 28g of ethyl 3-[(3-amino-4-methylaminobenzoyl)(pyridin-2-yl)amino]propionate and 14.5g of chloroacetic anhydride to the reactor at room temperature. , Stir and heat to 65°C for 2 hours, add 15g potassium carbonate to the reaction solution after cooling slightly, and keep at 65°C for 2 hours. After filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was cooled to 0°C with 150ml methyl tert-butyl ether to crystallize to obtain N-[[2-(chloromethyl)-1-methyl-1H-benzimidazole-5 -Yl]carbonyl]-N-2-pyridyl-β-alanine ethyl ester 29.8 g, yield 91%, product HPLC purity 98.0%.
[0049] (2) Condensation reaction
[0050] At room temperature, 1.5 g of sodium iodide, 8.0 g of sodium bicarbonate, and 0.75 g of tetrabutylammonium chloride were sequentially added to the reactor at room temperature, and then 50 ml of water and 65 ml of butyl acetate were added and stirred. After the solid is c...
Embodiment 3
[0056] (1) Closed loop reaction
[0057] Add 120ml of ethyl acetate, 28g of ethyl 3-[(3-amino-4-methylaminobenzoyl)(pyridin-2-yl)amino]propionate and 14.5g of chloroacetic anhydride to the reactor at room temperature. , Stir and heat to 65°C for 2 hours. After cooling slightly, add 12g of sodium carbonate and raise the temperature to 78°C for 1 hour. After filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was cooled to 0°C with 150ml methyl tert-butyl ether to crystallize to obtain N-[[2-(chloromethyl)-1-methyl-1H-benzimidazole-5 -Yl]carbonyl]-N-2-pyridyl-β-alanine ethyl ester 29.1 g, yield 89%, product HPLC purity 98.1%.
[0058] (2) Condensation reaction
[0059] At room temperature, 1.5 g of sodium iodide, 6.6 g of potassium carbonate, and 0.75 g of tetrabutylammonium bromide were sequentially added to the reactor at room temperature, and then 50 ml of water and 65 ml of ethyl acetate were added and stirred. After the solid is completely...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com