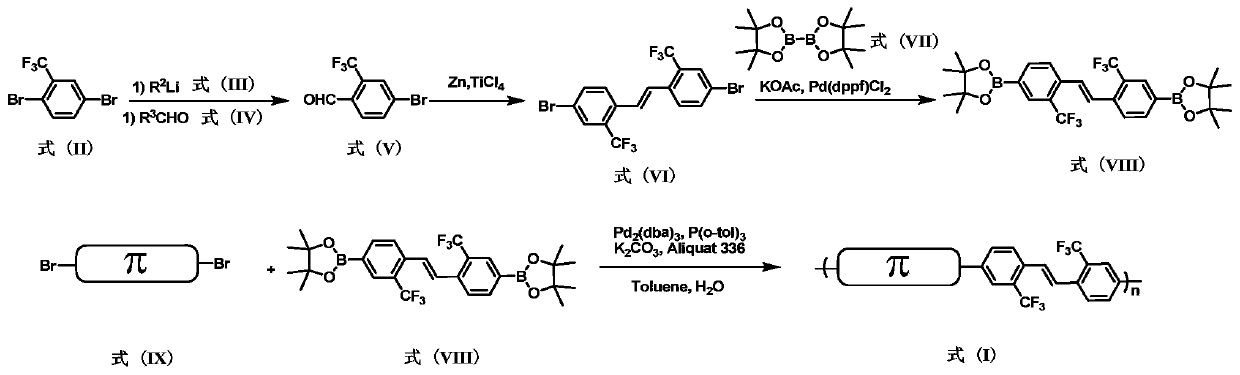
Trifluoromethyl-containing conjugated polymer, and preparation method and applications thereof
A technology of polymers and compounds, applied in the manufacture of semiconductor/solid-state devices, electrical components, circuits, etc., can solve the problems that are not enough to meet the structure-activity relationship and research of N-type semiconductor materials, achieve excellent thermal stability and fewer synthesis steps , The effect of simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
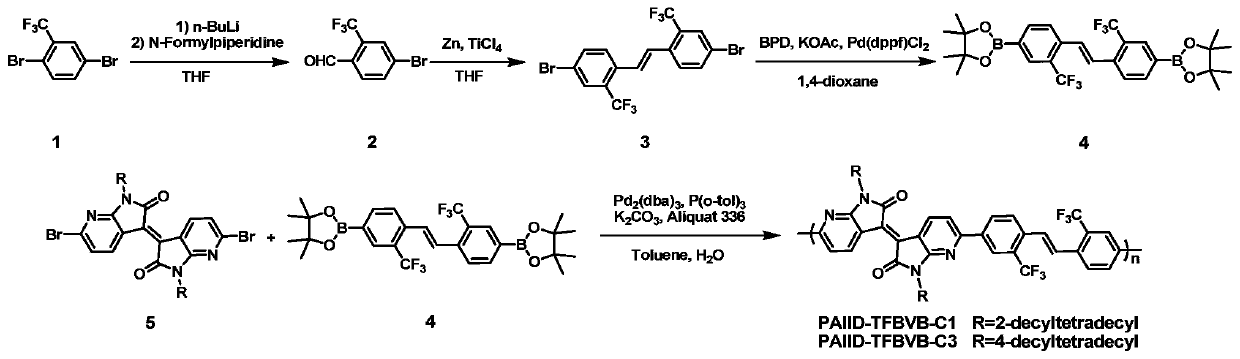
[0072] Embodiment 1, polymer PAIID-TFBVB-C1 synthesis (its synthetic route is as figure 2 shown)
[0073] 1) Synthesis of 2-(trifluoromethyl)-4-bromo-benzaldehyde (2)
[0074] 1,4-Dibromo-2-(trifluoromethyl)benzene (5.00 g, 16.45 mmol) and anhydrous tetrahydrofuran (120 ml) were successively added to a dry two-neck flask protected by argon. After the reaction system was cooled to -78°C, a 2.5M n-butyllithium solution in n-hexane (17.77 mmol, 7.11 mL) was slowly added dropwise. After the dropwise addition, continue stirring at -78°C for 10-20 minutes, then add N-formylpiperidine (2.79 g, 24.66 mmol) in one go, keep the reaction system at -78°C and stir for 30 minutes, Warm to room temperature slowly and stir the reaction overnight. The reaction was stopped, and the reaction solution was extracted with dichloromethane, dried over sodium sulfate and purified by column chromatography to obtain 3.50 g of the target product. Yield: 84%.
[0075] The structural characterization...
Embodiment 2
[0093] Embodiment 2, polymer PAIID-TFBVB-C3 synthesis (its synthetic route is as figure 2 shown)
[0094] Compound 5 (R=4-decyltetradecyl, 109.53 mg, 0.10 mmol) and compound 4 (56.82 mg, 0.10 mmol), tris(dibenzylideneacetone) dipalladium (4.50 mg), tris (o-Tolyl)phosphine (12.30 mg), chlorobenzene (5.0 mL), water (1.5 mL), potassium carbonate (138.21 mg, 1.0 mmol), and a catalytic amount of methyltrioctylammonium chloride were added under argon In a protected Schlenk bottle, the reaction system was refrigerated and deoxygenated at -78°C for 30 minutes, then stirred and reacted at 85°C for 72 hours, and phenylboronic acid and bromobenzene were successively added to the reaction system for capping. Stop the reaction and cool to room temperature, pour the reaction system into methanol and stir for 3 hours, then filter. The obtained product was purified with a Soxhlet extractor, and the solvents used were methanol, acetone, and n-hexane for 12 hours respectively. Finally, 133....
Embodiment 3
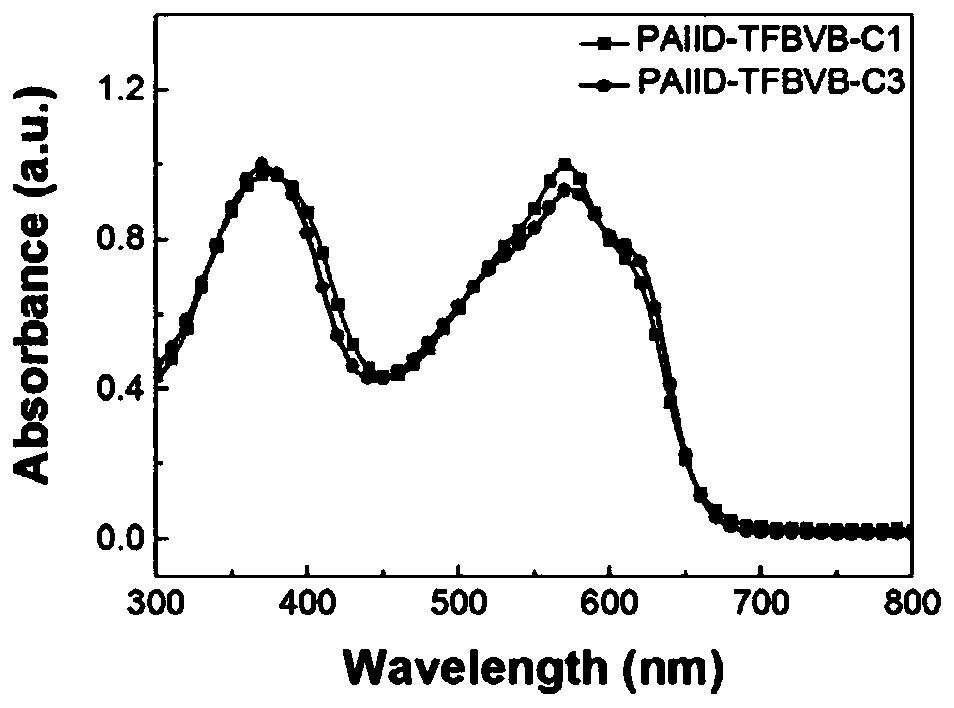
[0099] Spectral properties of embodiment 3, polymer PAIID-TFBVB-C1 and PAIID-TFBVB-C3
[0100] image 3 and Figure 4 The UV absorption spectrum of the polymer PAIID-TFBVB-C1 and PAIID-TFBVB-C3 chloroform solutions and films prepared in Examples 1 and 2.
[0101] Depend on image 3 It can be seen that this type of polymer exhibits strong double absorption bands in the ultraviolet region. while contrast image 3 , 4 It can be seen that the ultraviolet spectra of PAIID-TFBVB-C1 and PAIID-TFBVB-C3 do not change significantly from the solution to the film state, indicating that there is a strong interaction between the molecules in the solution state. In addition, the results also show that changing the alkyl side chain has little effect on the absorption spectrum.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com