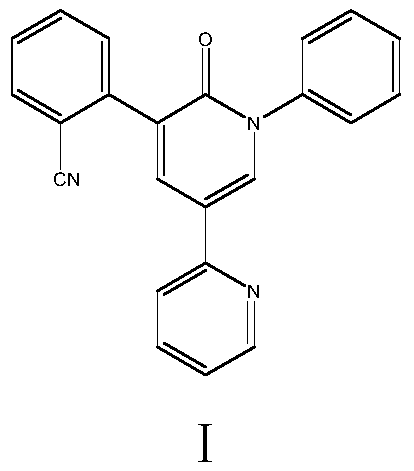
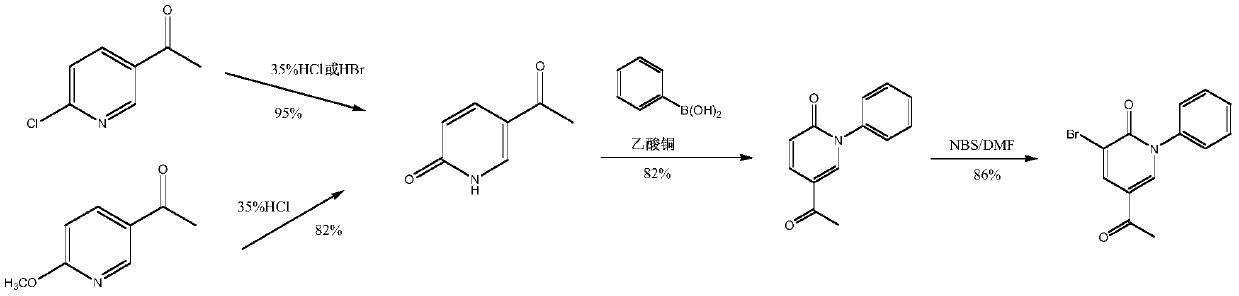
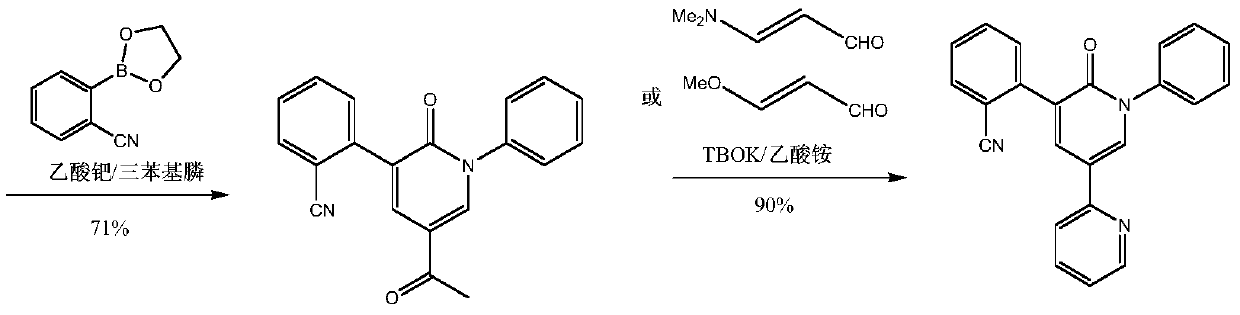
A kind of convenient preparation method of perampanel
A pyridine and compound technology, which is applied in the field of simple preparation of perampanel, can solve the problems of high environmental hazard, complicated operation, low yield and the like, and achieves high reaction atom economy, high yield and purity, and simple process operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: 3-Anilino-2-(pyridin-2-yl)propionitrile (II 1 , L=CN) Preparation
[0070] Into a 500 ml four-necked flask connected with a stirring, thermometer and reflux condenser, add 50 g methanol, 80 g water, 11.8 g (0.1 mol) pyridine-2-acetonitrile, 5.0 g (0.17 mol) paraformaldehyde, 10.0 G (0.11 mol) of aniline, 0.2 g of 30% hydrochloric acid, stirred at 70-72°C for 5 hours, cooled to 20-25°C, extracted with dichloromethane 3 times, 50 g of dichloromethane each time, combined the dichloromethane phases, 10.0 g of 5% sodium bicarbonate aqueous solution was washed once, and the solvent was recovered by distillation to obtain 22.1 g of light yellow viscous liquid 3-anilino-2-(pyridin-2-yl)propionitrile (Ⅱ) 1 ), the yield is 99.1%, and the gas phase purity is 99.8%.
Embodiment 2
[0071] Example 2: 3-Anilino-2-(pyridin-2-yl)propionitrile (II 1 , L=CN) Preparation
[0072] Into a 500 ml four-necked flask connected with a stirring, thermometer, and reflux condenser, add 20 g of ethanol, 70 g of water, 11.8 g (0.1 mol) of pyridine-2-acetonitrile, 20.0 g (0.2 mol) of 30% formaldehyde aqueous solution, 10.0 g (0.11 mol) of aniline, 0.1 g of 70% phosphoric acid, stirred at 80~82℃ for 4 hours, cooled to 20~25℃, extracted with dichloromethane 3 times, 50 g of dichloromethane each time, combined the dichloromethane phases , 10.0 g of 5% sodium bicarbonate aqueous solution was washed once, and the solvent was recovered by distillation to obtain 21.9 g of pale yellow viscous liquid 3-anilino-2-(pyridin-2-yl)propionitrile (Ⅱ) 1 ), the yield is 98.2%, and the gas phase purity is 99.9%.
Embodiment 3
[0073] Example 3: N-phenyl-N-[2-cyano-2-(pyridin-2-yl)]ethyl-2-cyanobenzeneacetamide (IV 1 ) Preparation
[0074] Into a 500 ml four-necked flask connected with a stirring, thermometer and distillation system, add 100 g of N,N-dimethylformamide and 22.5 g (0.1 mole) of 3-anilino-2-(pyridin-2-yl) Propionitrile (Ⅱ 1 ), 19.3 g (0.11 mol) of methyl 2-cyanophenylacetate, reacted at 100-105°C for 4 hours, and the removed methanol is distilled off at the same time, and the gas phase detection reaction is completed. N,N-dimethylformamide was recovered by distillation under reduced pressure, the temperature was lowered to 50-60°C, 50 g of methyl tert-butyl ether was added, the temperature was reduced to room temperature, and the filter cake was washed with 20 g of methyl tert-butyl ether and dried. 34.3 g of N-phenyl-N-[2-cyano-2-(pyridin-2-yl)]ethyl-2-cyanobenzeneacetamide (IV 1 ), the yield is 93.5%, and the gas phase purity is 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com