Monoclonal antibody resisting XOD (xanthine oxidase) and preparation method and application thereof
A technology of xanthine oxidase and monoclonal antibody, which is applied in the field of pharmacy, medicine and immunology, can solve the problems of antibody difficulty and poor effect, and achieve the effect of enhancing immunogenicity, broad application prospect and important theoretical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of peptide immunogen or antigenic peptide for immunization:
[0043]Connect the C-terminal of the IA2(5) peptide FEYQD (see SEQ ID NO.1 for the amino acid sequence) and the N-terminal of the Th2 epitope P2 peptide (see SEQ ID NO.2 for the amino acid sequence) to form a carrier through a flexible peptide or a connecting peptide The peptide, named IA2(5)-P2-1 (abbreviated as IP) and the B cell epitope O of human xanthine oxidase (indicated by B, O is a polypeptide sequence of xanthine oxidoreductase 1101RLEPYKKKNPS1111, a total of 11 amino acid residues, the amino acid sequence is shown in SEQ ID NO.3), and the C-terminus is covalently linked by a flexible peptide or a connecting peptide to form a peptide immunogen B-IA2(5)-P2-1 (this patent is O-IA2(5) )-P2-1, the amino acid sequence is shown in SEQ ID NO.4), the peptide immunogen or antigenic peptide is synthesized by FMOC solid-phase synthesis method, and the purity detected by HPLC is >90%. The...
Embodiment 2
[0044] Embodiment 2: Preparation of animal immunization and hybridoma cells
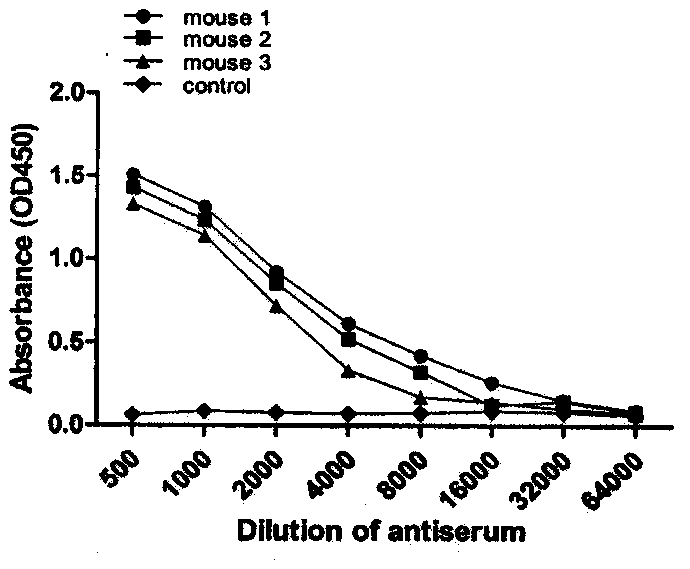
[0045] Animal immunization and preparation of immune spleen lymphocytes: the above antigenic peptides were emulsified with QuickAntibody-Mouse5W adjuvant, mixed with 0.9% saline for injection 1:1, and immunized 6-8 weeks old BALB / c mice by intramuscular injection. The immunization dose was 100 μg / rat, and the second immunization was carried out with the same immunization method and dose two weeks later. After two immunizations, the orbital blood was taken to determine the serum titer by ELISA gradient dilution, and the immunized spleen lymphocytes of the mouse with the highest antibody titer were selected for cell fusion. Conclusion: The above-mentioned antigenic peptides produced higher titers (greater than 1:10000) after immunizing mice, and could be used for the preparation of monoclonal antibodies ( figure 1 ).
[0046] Cell fusion: Sp2 / 0 derived from BALB / c mice was used for myeloma cells, and...
Embodiment 3
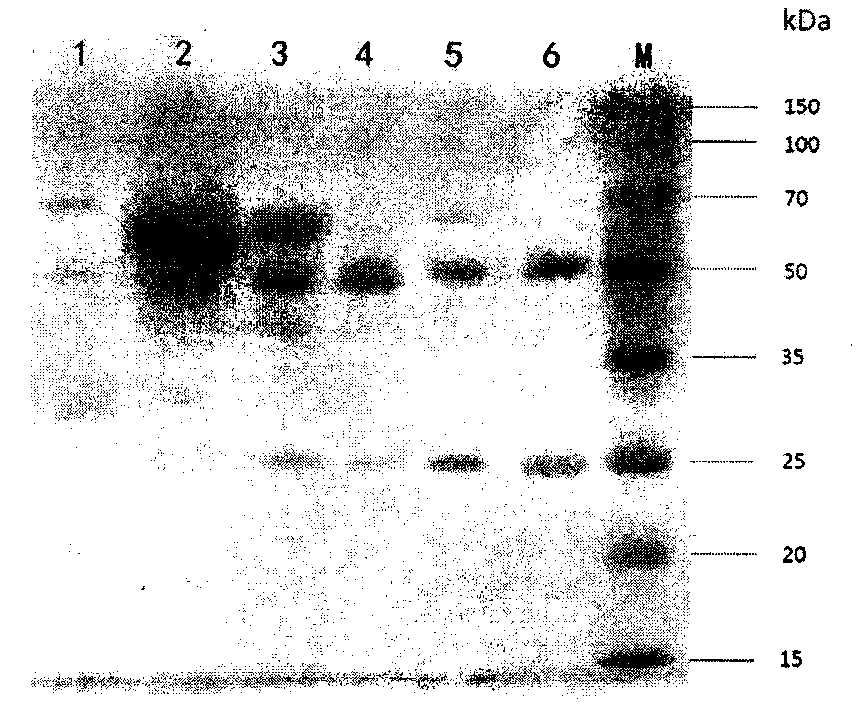
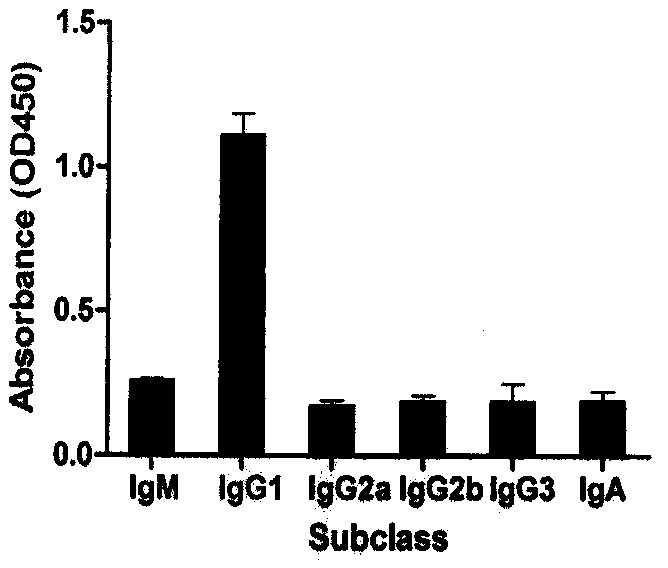
[0048] Example 3: Preparation and purification of monoclonal antibody mAb (named CPUKO1)
[0049] Preparation of mouse monoclonal antibody ascites: the method of producing monoclonal antibody in animals is adopted. Each Balb / c mouse was intraperitoneally injected with 0.5ml of sterilized liquid paraffin, and after 7 days, each mouse was intraperitoneally injected with 1×10 6 mouse hybridoma cells. After 7 days, observe the production of ascites in the mice. If the abdomen is obviously enlarged, the ascites can be extracted. Ascitic fluid contains red blood cells, cell debris, fibrin clots, and lipids. First remove the precipitated cells by low-speed centrifugation, then high-speed centrifugation to remove cell residues and small particles, and use a 0.2 μm microporous membrane to remove fibrin clots and lipids.
[0050] Caprylic acid-ammonium sulfate precipitation method: the initially purified ascitic fluid was crudely extracted with caprylic acid-ammonium sulfate precipit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com