A Peptide Fluorescent Probe Specifically Binding to Copper Ion and Cysteine
A fluorescent probe, species-specific technology, applied in the field of analysis and detection, can solve the problems of low probe sensitivity, poor biocompatibility, complicated preparation, etc., and achieve the effect of good biocompatibility, low toxicity, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Polypeptide and polypeptide fluorescent probe P and polypeptide fluorescent probe P-Cu
[0035] 1.1 Preparation method
[0036] (1) Add 100 mg of Rink ammonia resin to a reactor with a sieve plate, use 5 mL of dichloromethane to swell the resin; remove N-terminal Fmoc from 20% piperidine / N,N-dimethylformamide (DMF) solution Protecting group, chromogenic reaction detects that the protecting group is completely removed;
[0037] (2) Dissolve 4eq N-terminal Fmoc-protected amino acids with 4eq HOBt and 4eq HBTU in DMF, activate at low temperature for 20min, add 6eq DIEA dropwise to the solution, mix the solution and add it to the reactor, and react for 3hrs;
[0038](3) After the reaction was finished, the reaction liquid was extracted from the reactor, and the resin was washed three times with 5 mL of DMF and DCM respectively. The complete condensation of amino acids was detected by color reaction, and the resin was treated with 20% piperidine / DMF solution for ...
Embodiment 2
[0046] Example 2 Polypeptide fluorescent probe P to Cu 2+ Specificity studies of detection
[0047] 2.1 Evaluation experiment of cation selectivity of polypeptide fluorescent probe P
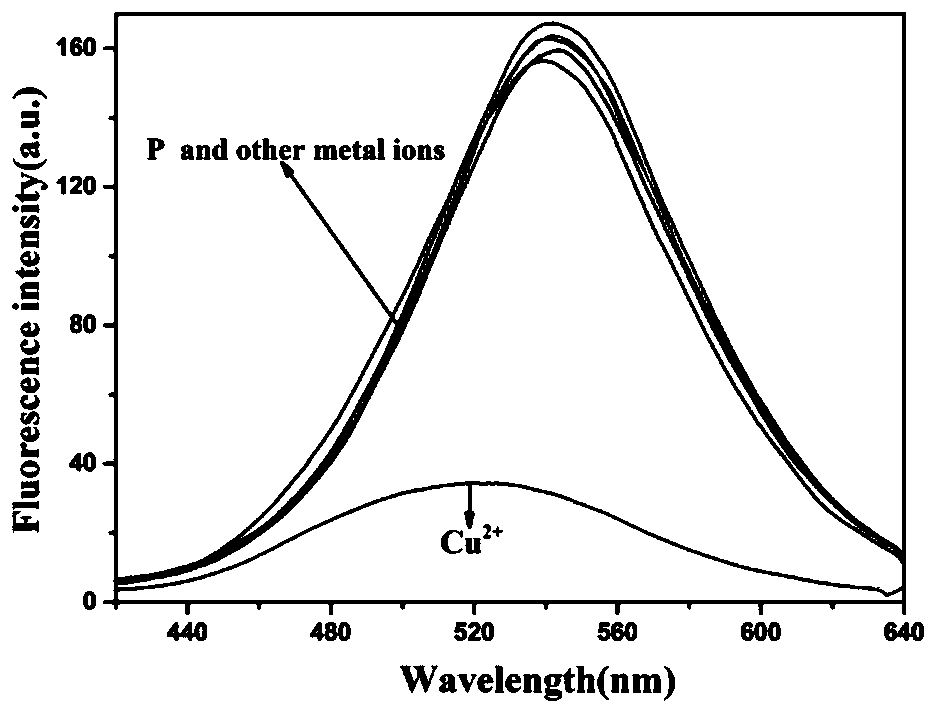
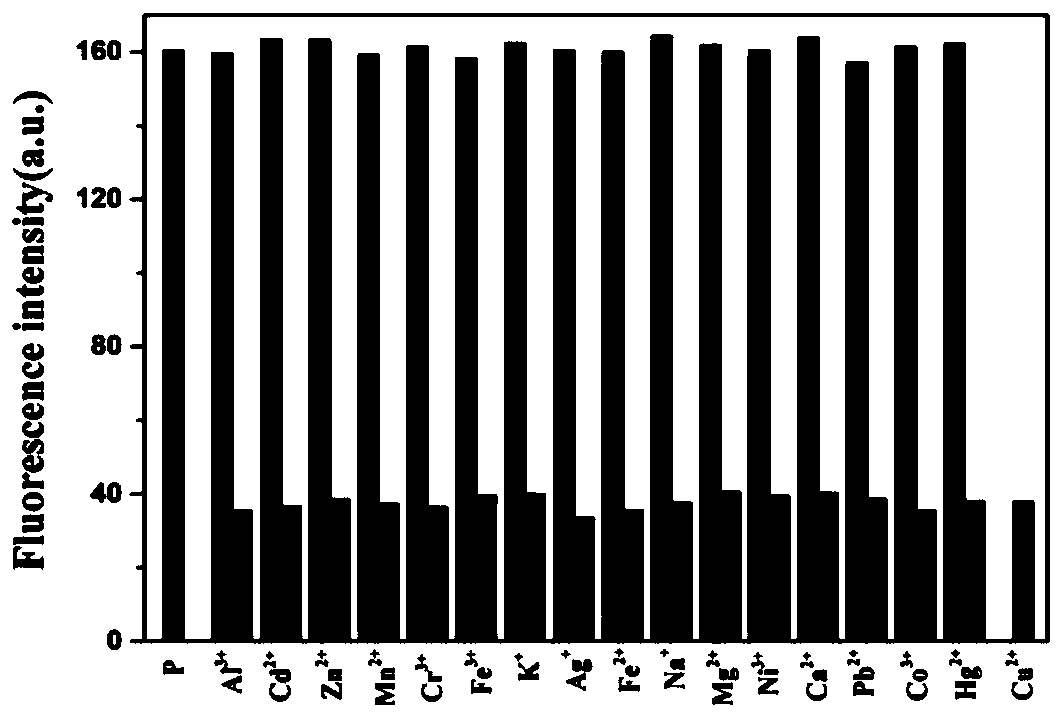
[0048] In HEPES buffer (10mM, pH7.4), add 10 -5 mol / L polypeptide fluorescent probe P and 10 -5 mol / L metal cation (Ag + , Al 3+ , Ca 2+ , Cd 2+ ,Co 2+ , Cr 3+ , Cu 2+ , Fe 3+ , Hg 2+ , K + , Mg 2+ , Mn 2+ , Na + , Ni 2+ , Pb 2+ ), to detect the change in the fluorescence emission spectrum of the solution. The result is as figure 2 As shown, only Cu 2+ In the presence of , the fluorescence of the probe P was significantly quenched, and the other 15 metal ions could not quench the fluorescence of the polypeptide fluorescent probe P, indicating that the polypeptide should be able to recognize Cu 2+ .
[0049] 2.2 Polypeptide fluorescent probe P versus Cu2 + Specific detection research
[0050] In HEPES buffer (10mM, pH7.4), add 10 -5 mol / L polypeptide fluorescent probe P a...
Embodiment 3
[0057] Example 3 Specificity Research of Polypeptide Fluorescent Probe P-Cu for Cysteine (Cys) Detection
[0058] 3.1 Selectivity evaluation experiment of polypeptide fluorescent probe P-Cu for amino acid detection
[0059] To HEPES buffer (10mM, pH7.4), add 10 -5 mol / L peptide fluorescent probe P-Cu and 10 - 5 mol / L cysteine (Cys), glutamic acid (Glu), aspartic acid (Asp), asparagine (Asn), phenylalanine (Phe), alanine (Ala), gluten Aminoamide (Gln), Threonine (Thr), Serine (Ser), Tyrosine (Tyr), Lysine (Lys), Tryptophan (Trp), Proline (Pro), Glycine (Gly) , valine (Val), methionine (Met), leucine (Leu), isoleucine (Ile), arginine (Arg), histidine (His), glutathione (GSH) and S 2- , to detect changes in the fluorescence emission spectrum of the solution. The result is as Figure 6 As shown, only the addition of Cys causes the fluorescence intensity to become significant, while other substances do not cause significant changes in fluorescence intensity.
[0060] 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com