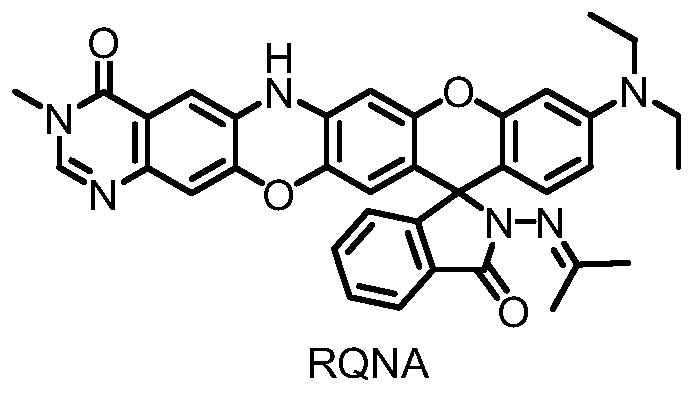
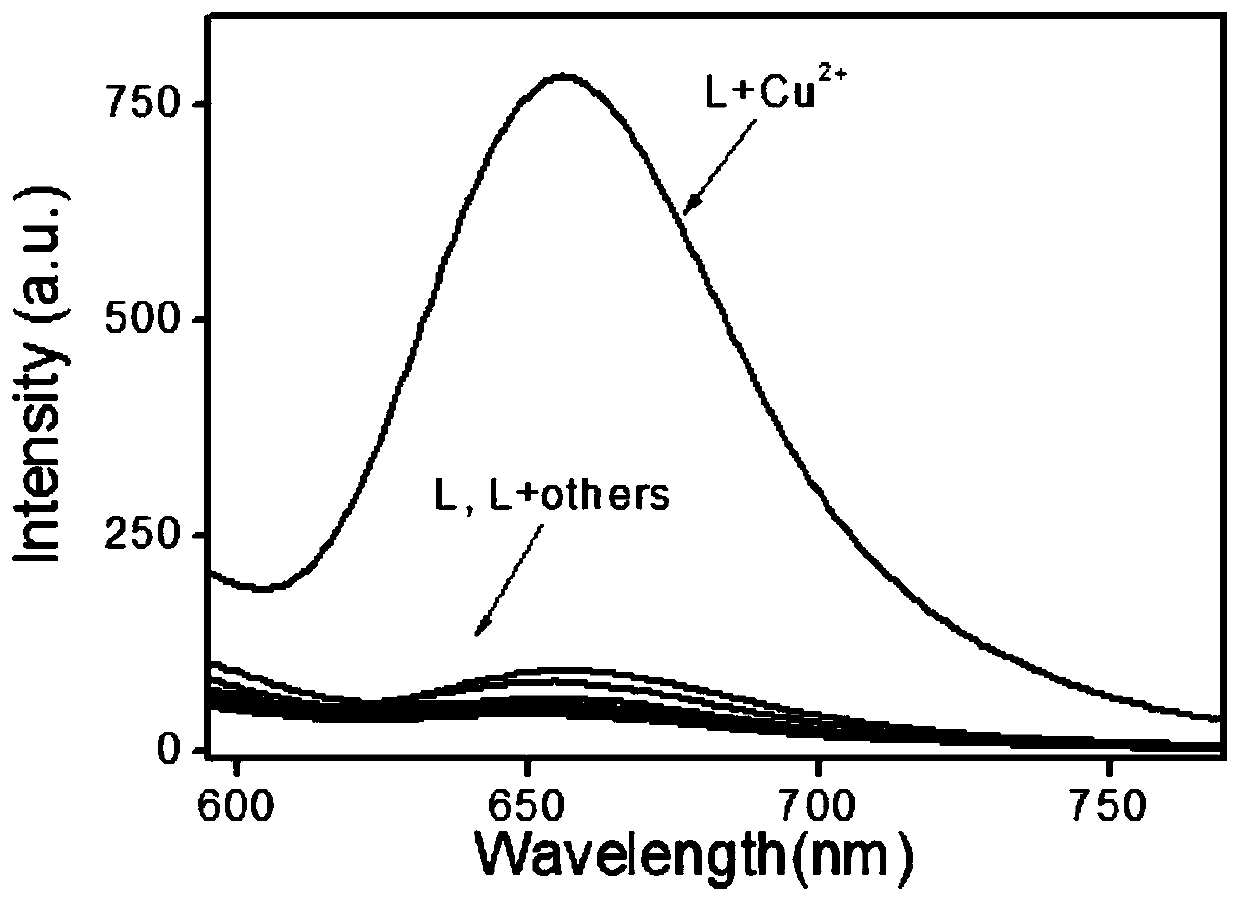
Large Stokes shift dark red fluorescent probe RQNA for detecting copper ions, and preparation method and application thereof
A copper ion detection and Stokes technology, applied in the field of molecular fluorescent probes, can solve problems such as rare probes, and achieve the effects of good cell membrane permeability, low cytotoxicity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the preparation route of compound B are as follows:
[0024]
[0025] Concrete preparation steps are as follows:
[0026] 1) Change CH 3 I (30mmol, 4.26g) was added to a mixture of 7-chloro-6-nitroquinazolin-4(3H)-one (20mmol, 4.5g) and anhydrous potassium carbonate (60mmol, 8.28g) in methanol (50mL) solution, and then reacted at 65°C for 22 hours, and cooled to room temperature after the reaction to obtain a reaction solution;
[0027] 2) The above reaction solution was poured into 100 mL of water and extracted with dichloromethane (3×100 mL). The organic phases were combined and collected, dried over anhydrous sodium sulfate, and then concentrated by vacuum distillation to obtain a crude product. The crude product was separated and purified by column chromatography on silica gel, and the eluent used was a dichloromethane-methanol mixture with a volume ratio of 25:1-2 to obtain light yellow solid Compound B with a yield of 89%; melting point: 208 -...
Embodiment 2
[0029] Embodiment 2, the preparation route of compound C are as follows:
[0030]
[0031] Concrete preparation steps are as follows:
[0032] 1) Dissolve rhodanol isomers (2.2mmol, 1688.6 mg) and compound B (2mmol, 478mg) in DMF (6mL), then react at 80°C for 12 hours, cool to room temperature after the reaction to obtain a reaction solution ;
[0033] 2) The above reaction solution was poured into 100 mL of water and extracted with dichloromethane (3×50 mL). The organic phases were combined and collected, dried over anhydrous sodium sulfate, and then concentrated by vacuum distillation to obtain a crude product. The crude product was separated and purified by column chromatography on silica gel, and the eluent used was a mixture of petroleum ether-ethyl acetate-methanol with a volume ratio of 20:6:1 to obtain compound C as a yellow solid with a yield of 81%; : 168-172°C.
[0034] 1 H NMR (CDCl 3,400MHz,ppm)δ=8.76(1H),8.02(1H),7.95(1H),7.66(1H),7.58(1H),7.32(1H),7.21(...
Embodiment 3
[0035] Embodiment 3, the preparation route of compound D are as follows:
[0036]
[0037] Concrete preparation steps are as follows:
[0038] 1) Add 1mL of concentrated hydrochloric acid to a mixed methanol solution (20mL) of compound C (2mmol, 1.18g) and tin protochloride (10mmol, 2.25g), then react at 65°C for 12 hours, and cool to room temperature after the reaction get the reaction solution;
[0039] 2) The above reaction solution was poured into 100 mL of water and extracted with dichloromethane (3×50 mL). The organic phases were combined and collected, dried over anhydrous sodium sulfate, and then concentrated by vacuum distillation to obtain a crude product. The crude product was separated and purified by column chromatography on silica gel, and the eluent used was a dichloromethane-methanol mixture with a volume ratio of 50:1-2 to obtain compound D as a yellow solid with a yield of 77%; melting point: 178- 182°C.
[0040] 1 H NMR (CDCl 3 ,400MHz,ppm)δ=8.25(1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com