Near-infrared fluorescent probe responding to peroxynitroso anions, and preparation method and application thereof
A technology of peroxynitroso and fluorescent probes, applied in the field of biochemistry, can solve the problems of short half-life and unreported fluorescent probes, and achieve the effects of small light damage, saving reaction costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] A method for preparing a near-infrared fluorescent probe responsive to peroxynitrite anions, comprising the following steps:
[0045] (1) Put 2-R 1 -6-R 2- Add 4-nitrophenol to the first solvent, add sodium hydride and chloromethyl ether, and react at 20-40°C to obtain 1-(ethoxymethoxy)-2-R 1 -6-R 2 -4-nitrobenzene;
[0046] (2) 1-(ethoxymethoxy)-2-R 1 -6-R 2 -4-nitrobenzene was added to the second solvent, sodium sulfide nonahydrate was added, and the reaction was stirred at room temperature to obtain 4-(ethoxymethoxy)-3-R 1 -5-R 2 -aniline;
[0047] (3) 4-(ethoxymethoxy)-3-R 1 -5-R 2 - Add aniline to the third solvent, add sodium methoxide and paraformaldehyde, stir for 2 to 5 hours, then add sodium borohydride, heat up and react to obtain 4-(ethoxymethoxy)-3-R 1 -5-R 2 -N-Methylaniline;
[0048] (4) Add isophorone and malononitrile to the fourth solvent, add piperidine, acetic acid and acetic anhydride, stir at room temperature for 4-8 hours, then heat up...
Embodiment 1
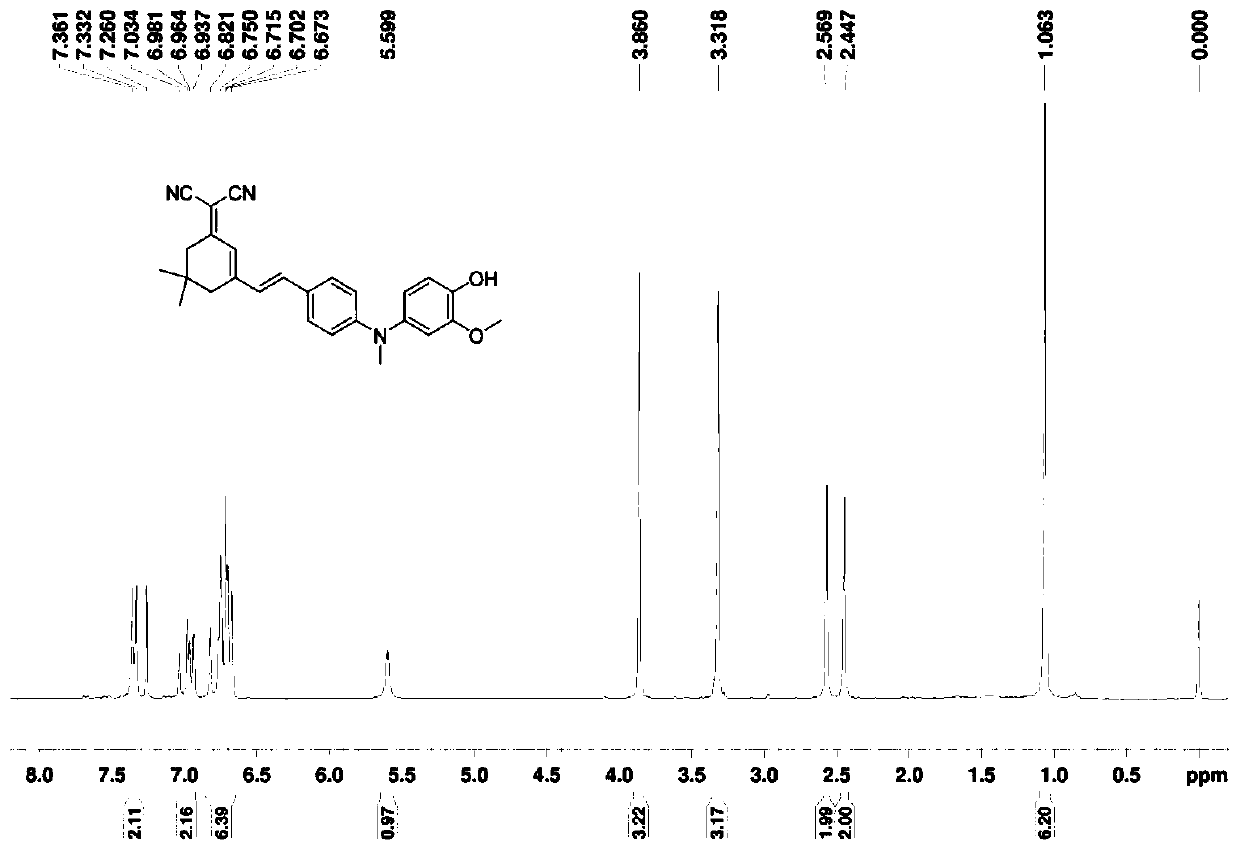
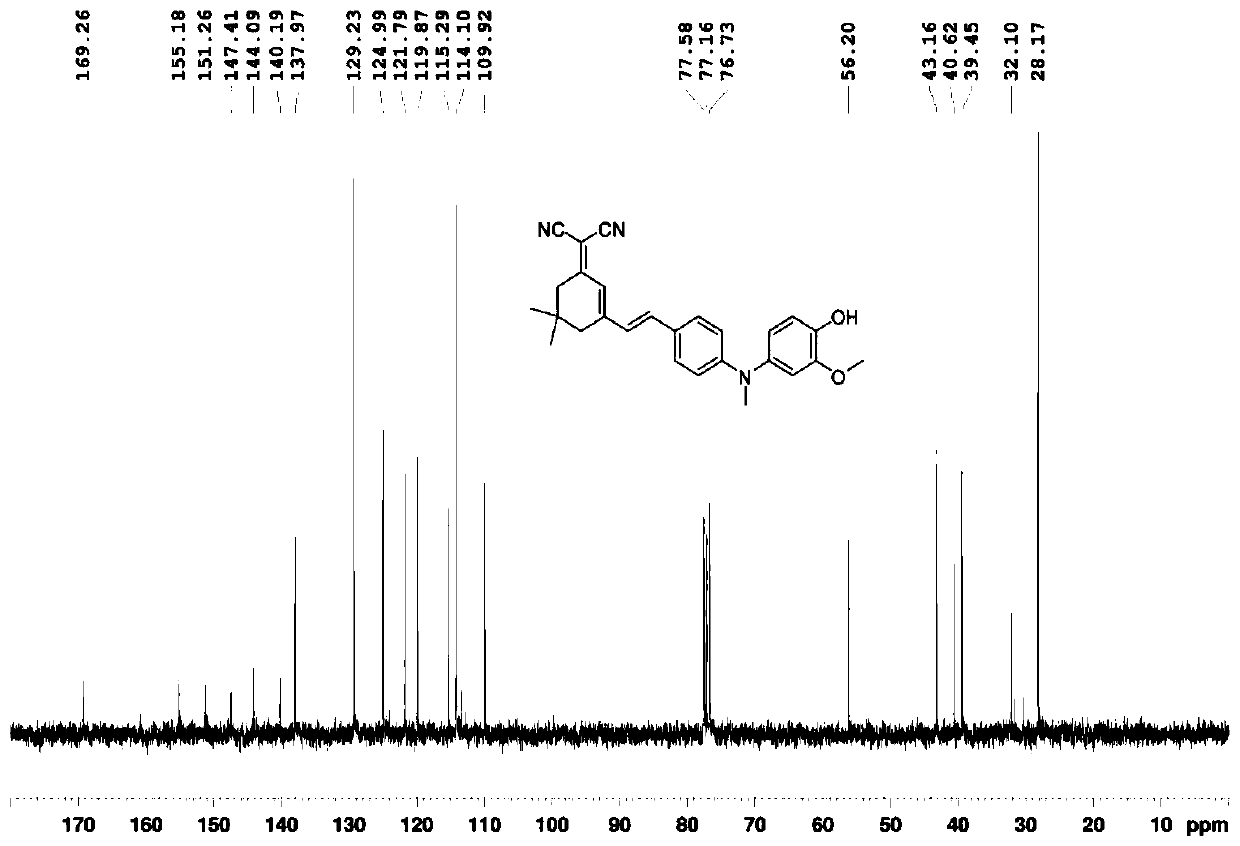
[0056] Add 2-methoxy-4-nitrophenol (5g, 29.6mmol) into the reactor, dissolve it in anhydrous tetrahydrofuran at 0°C, add sodium hydride (88.8mmol) and stir at 0°C for 0.5h, then add dropwise Chloromethyl ether (59.2 mmol), continued stirring for 1 h, transferred to room temperature and stirred for 3 h. After the reaction is finished, quench sodium hydride with ice water, extract with ethyl acetate, wash with water, dry over anhydrous magnesium sulfate, spin dry the solvent under reduced pressure, and use a developer of dichloromethane / petroleum ether with a volume ratio of 2:3 for column layer Analysis and separation, the target product was obtained with a yield of 95%.
[0057] Compound 3 (2g, 8.8mmol) was added to the reactor, dissolved in ethanol, sodium sulfide nonahydrate (44mmol) was added in batches, and stirred at room temperature for 8h. After the reaction was completed, the solvent was spin-dried under reduced pressure, extracted with ethyl acetate, washed with wate...
Embodiment 2
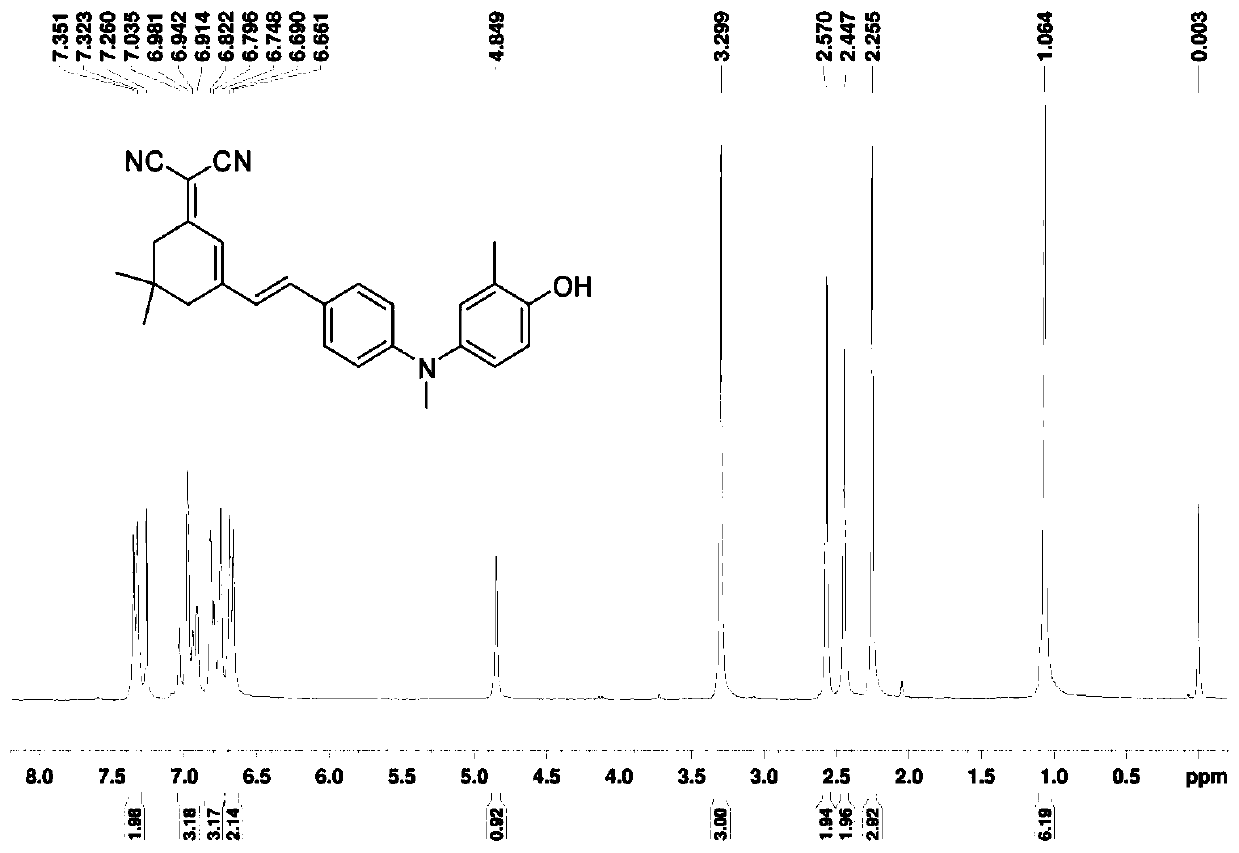
[0066] Add 2-methyl-4-nitrophenol (4.5g, 29.6mmol) into the reactor, dissolve in anhydrous tetrahydrofuran at 0°C, add sodium hydride (88.8mmol), stir at 0°C for 0.5h, then drop Add chloromethyl ether (59.2 mmol), continue stirring for 1 h, transfer to room temperature and stir for 3 h. After the reaction is finished, quench sodium hydride with ice water, extract with ethyl acetate, wash with water, dry over anhydrous magnesium sulfate, spin dry the solvent under reduced pressure, and use a developer of dichloromethane / petroleum ether with a volume ratio of 2:3 for column layer Analysis and separation, the target product was obtained with a yield of 93%.
[0067] Compound 4 (1.85g, 8.8mmol) was added to the reactor, dissolved in ethanol, sodium sulfide nonahydrate (44mmol) was added in batches, and stirred at room temperature for 8h. After the reaction was completed, the solvent was spin-dried under reduced pressure, extracted with ethyl acetate, washed with water, dried over...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com