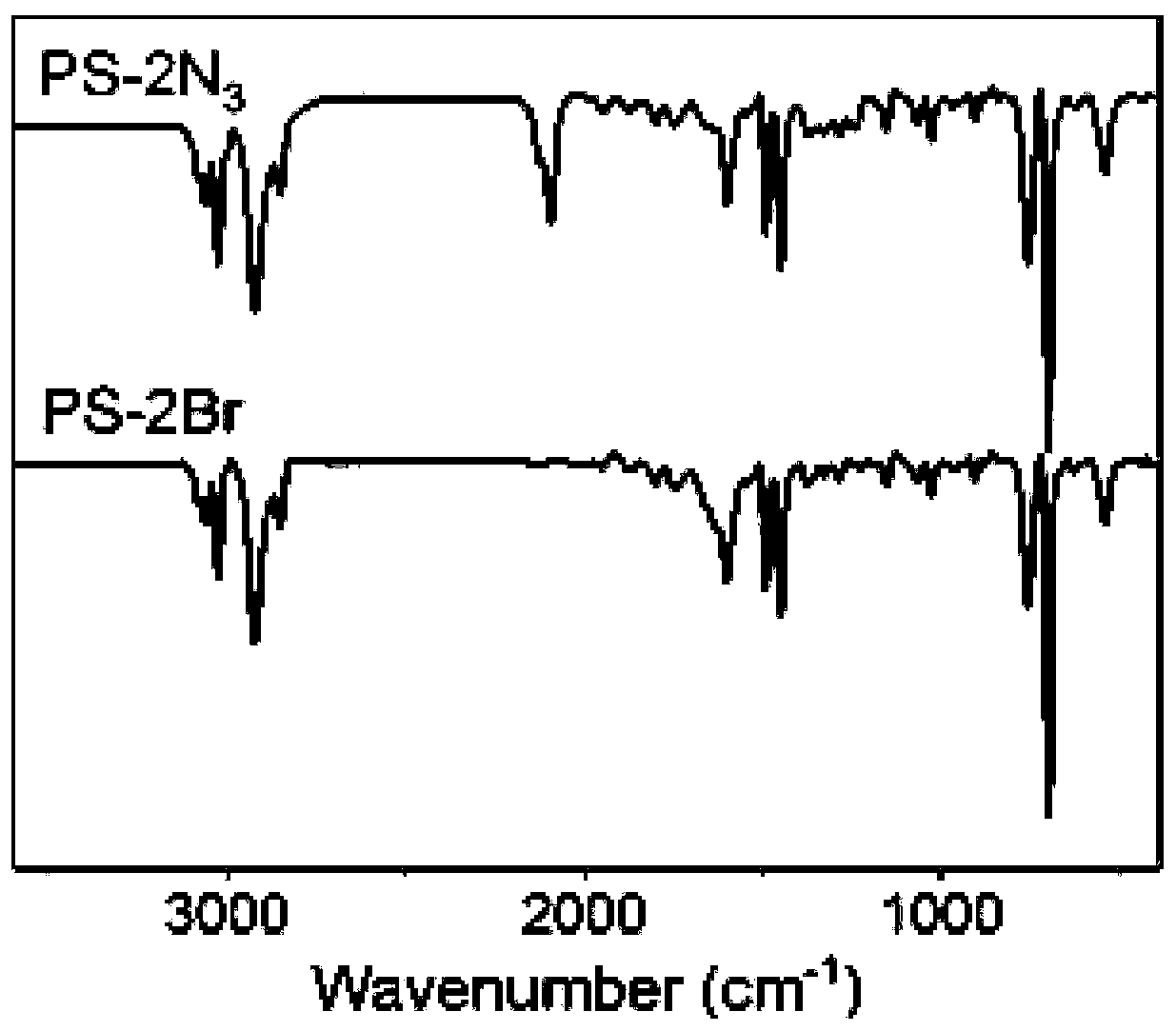
Polycaprolactone-based hyperbranched polymer all-solid-state electrolyte and lithium ion battery
A technology of hyperbranched polymer and polycaprolactone is applied in the field of lithium-ion batteries, which can solve the problems of instability of electrolyte and lithium metal, potential safety hazards of solvent flammability, and inability to support battery operation, and achieve excellent charge-discharge rate. performance, excellent long-term cycle stability, easy-to-implement effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
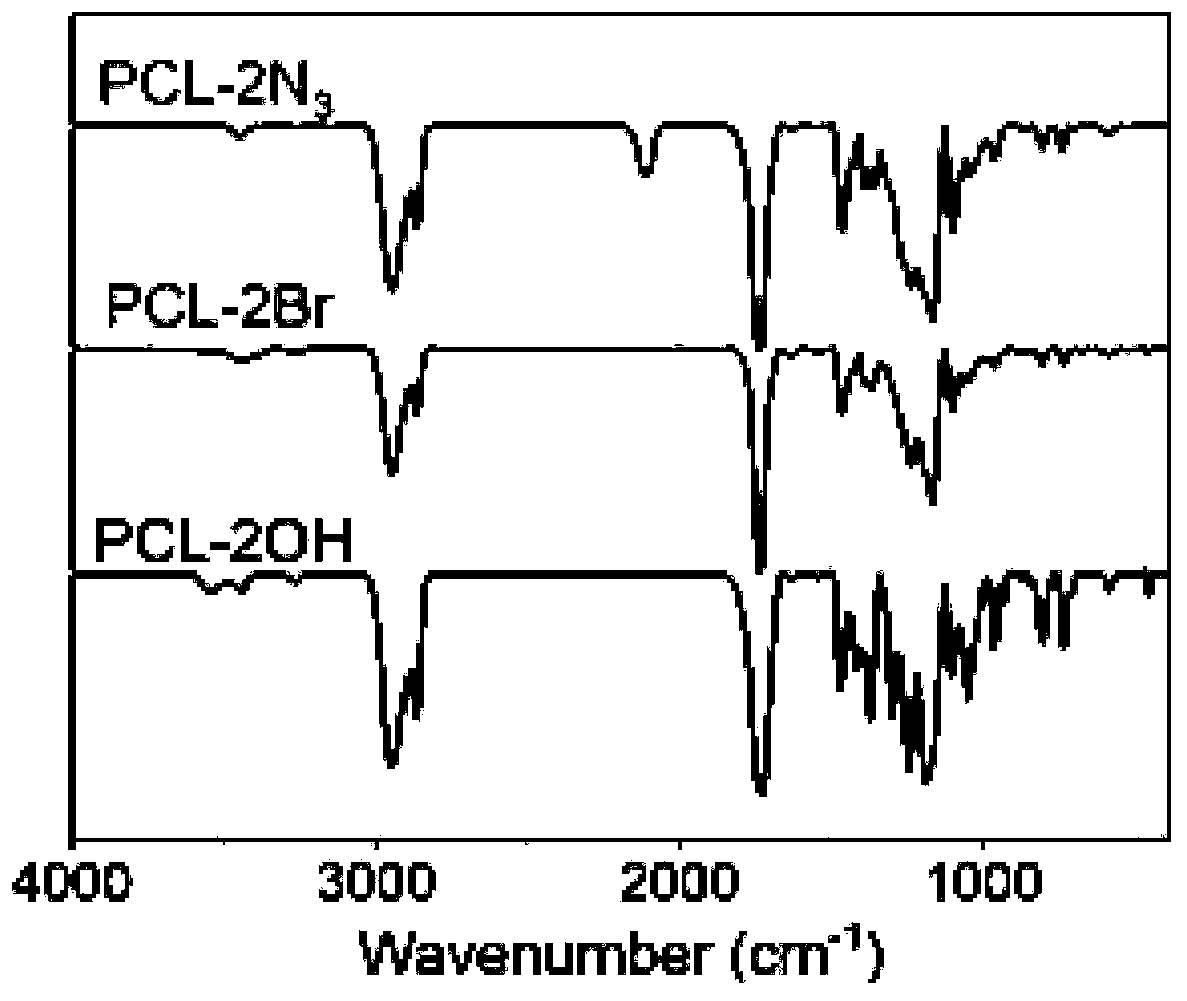
[0107] In a three-neck round bottom flask, 20 g of caprolactone monomer with molecular weight of 114, 0.84 g of initiator (2,2-bis((2'-hydroxy-2'-methylpropionyloxy)methyl)propane Propargyl ester), 0.1g of stannous octoate were added to 150ml of anhydrous toluene, fully dissolved to form a reaction mixture, the reaction temperature was 110°C, and the reaction time was 5h. After the reaction was complete, the solvent was removed by rotary evaporation and the product was redissolved in THF. The solution was precipitated three times in an excess of cold methanol / water mixture (2 / 1, v / v). After drying under vacuum for 24 hours, polycaprolactone (Alkyne-(PCL-OH) 2 ).
[0108] In a three-neck round bottom flask, 15g Alkyne-(PCL-OH) 2 Added to 200ml of anhydrous acetonitrile. Under a nitrogen atmosphere, add 2-bromoisobutyryl bromide (12.45 g) dropwise in 50 ml of anhydrous acetonitrile at 0°C, stir at 4°C for 24 hours after the addition, remove the solvent, and redissolve the pr...
Embodiment 2
[0118] According to the same method as in Example 1, the difference is that the weight ratio of polycaprolactone with both azido and alkynyl end caps to polystyrene with both azide and alkynyl end caps is 7:3, to prepare the whole solid electrolyte membrane.
[0119] In an argon glove box, the obtained all-solid-state electrolyte membrane was cut into 18mm discs, and stainless steel was used as a blocking electrode to assemble a button cell. The AC impedance test was carried out at room temperature, and the measured room temperature conductivity was 7.86×10 -7 S / cm. The prepared composite organic solid electrolyte uses lithium sheets and stainless steel sheets as electrodes to assemble button batteries, and conducts linear sweep voltammetry tests. The electrochemical stability window at room temperature can reach 5.6V. The prepared composite organic solid electrolyte was assembled into a button battery using lithium sheets as symmetrical electrodes, and the chronoamperometry ...
Embodiment 3
[0121] According to the same method as in Example 1, the difference is that the weight ratio of polycaprolactone having both azido and alkynyl terminations to polystyrene having both azido and alkynyl terminations is 5:5, to prepare the whole solid electrolyte membrane.
[0122] In an argon glove box, the obtained all-solid electrolyte membrane was cut into 18mm discs, and stainless steel was used as a blocking electrode to assemble a button cell. The AC impedance test was carried out at room temperature, and the measured room temperature conductivity was 1.59×10 -6 S / cm. The prepared composite organic solid electrolyte uses lithium sheets and stainless steel sheets as electrodes to assemble button batteries, and conducts linear sweep voltammetry tests, and its electrochemical stability window can reach 6.0V at room temperature. The prepared composite organic solid electrolyte was assembled into a button battery using lithium sheets as symmetrical electrodes, and the chronoam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity at room temperature | aaaaa | aaaaa |
| Conductivity at room temperature | aaaaa | aaaaa |
| Conductivity at room temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com