A kind of ketorolac tromethamine injection that reduces irritation and does not contain organic solvents
A technology of ketorolac tromethamine and ketorolac tromethamine, which is applied in the field of ketorolac tromethamine injection, can solve the problems of patients with strong pain, vasospasm, and obvious pain, and achieve insoluble particles and Reduced white spots, reduced risk of vascular embolism, and improved overall quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
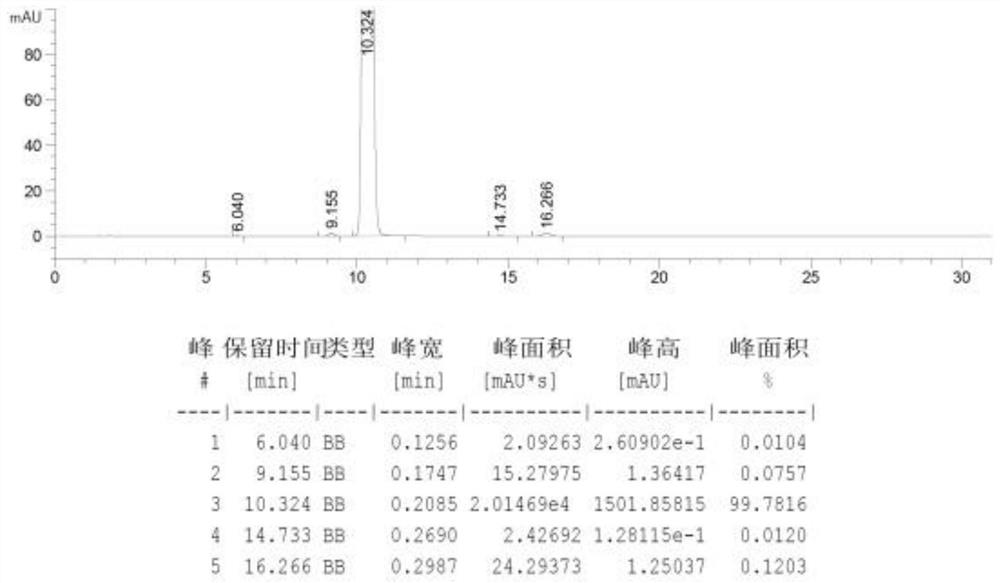
Examples
Embodiment 1
[0095] A: Prescription composition
[0096] Ketorolac tromethamine 10g trometamol 11.78g Sodium chloride 2.70g hydrochloric acid Appropriate amount, adjusted to pH about 7.3 Water for Injection to 1L batch 1000 pieces
[0097] B: Preparation method:
[0098] 1. Add tromethamine and sodium chloride into 95% prescription water for injection cooled to room temperature, and stir until completely dissolved;
[0099] 2. Add ketorolac tromethamine and stir until completely dissolved;
[0100] 3. Add 1M hydrochloric acid aqueous solution to adjust the pH of the liquid to about 7.26;
[0101] 4. Use the remaining amount of water for injection to adjust the concentration of ketorolac tromethamine to 10 mg / mL;
[0102] 5. The liquid medicine is filtered through a 0.2μm filter element to the buffer tank of the filling machine;
[0103] 6. Under the protection of nitrogen, fill the liquid medicine into the cleaned and dry heat sterilized gla...
Embodiment 2
[0108] A: Prescription composition
[0109]
[0110]
[0111] B: Preparation method:
[0112] 1. Add tromethamine and sodium chloride into 95% prescription water for injection cooled to room temperature, and stir until completely dissolved;
[0113] 2. Add ketorolac tromethamine and stir until completely dissolved;
[0114] 3. Add 1M hydrochloric acid aqueous solution to adjust the pH of the liquid to about 7.29;
[0115] 4. Use the remaining amount of water for injection to adjust the concentration of ketorolac tromethamine to 15 mg / mL;
[0116] 5. The liquid medicine is filtered through a 0.2μm filter element to the buffer tank of the filling machine;
[0117] 6. Under the protection of nitrogen, fill the liquid medicine into the cleaned and dry heat sterilized glass ampoule, and seal it;
[0118] 7. Moist heat sterilization, F 0 ≥12;
[0119] 8. Inspection and elimination of unqualified products;
[0120] 9. Outsource the labeling of qualified products.
Embodiment 3
[0122] A: Prescription composition
[0123] Ketorolac tromethamine 30g trometamol 2.46g Sodium chloride 4.03g hydrochloric acid Appropriate amount, adjusted to pH about 7.3 Water for Injection to 1L batch 1000 pieces
[0124] B: Preparation method:
[0125] 1. Add tromethamine and sodium chloride into 95% prescription water for injection cooled to room temperature, and stir until completely dissolved;
[0126] 2. Add ketorolac tromethamine and stir until completely dissolved;
[0127] 3. Add 1M hydrochloric acid aqueous solution to adjust the pH of the liquid to about 7.32;
[0128] 4. Use the remaining amount of water for injection to adjust the concentration of ketorolac tromethamine to 30mg / mL;
[0129] 5. The liquid medicine is filtered through a 0.2μm filter element to the buffer tank of the filling machine;
[0130] 6. Under the protection of nitrogen, fill the liquid medicine into the cleaned and dry heat sterilized gla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com