Preparation method of isovaleraldehyde
A technology of isovaleraldehyde and isoamyl alcohol, applied in the field of chemical industry, can solve the problems of many side reactions, cumbersome operation, complicated process, etc., and achieve the effects of increasing specific surface and mechanical strength, avoiding dehydration reaction, and increasing reaction concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
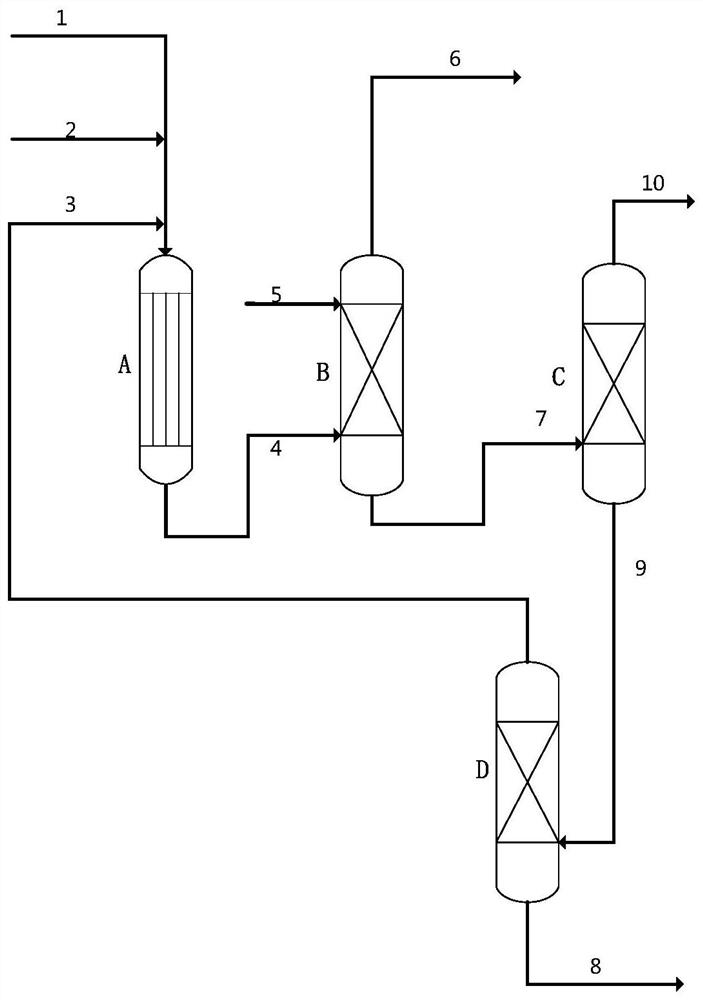
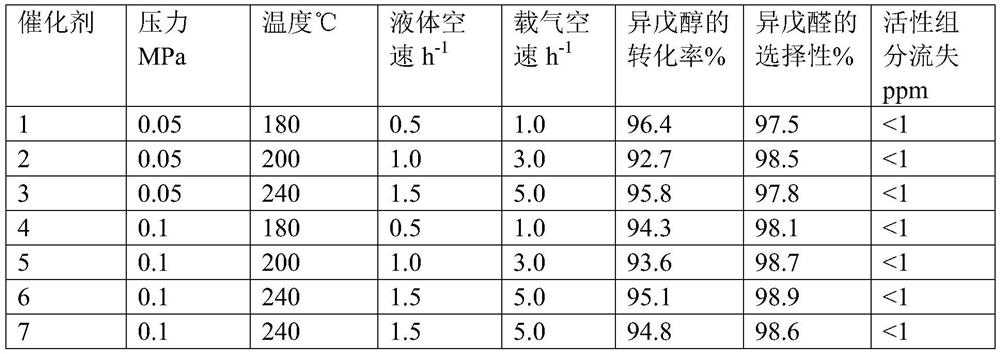
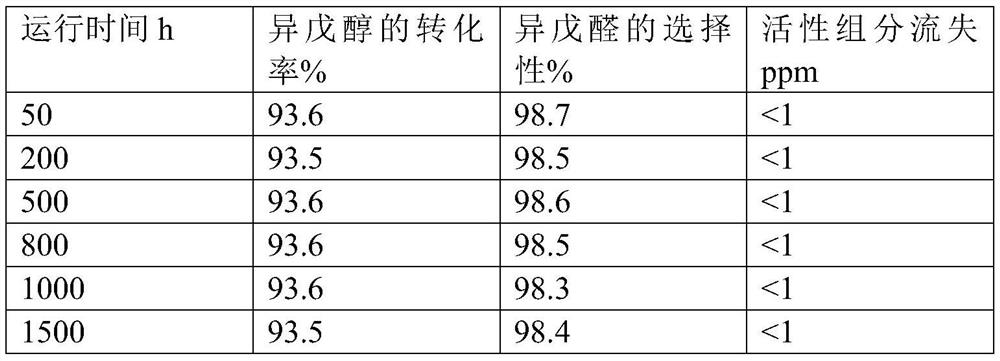
Image
Examples
Embodiment 1
[0035]Dissolve 125.5g of cuprous nitrate and 18.9g of zinc nitrate in 1000g of distilled water to obtain a mixed salt solution, raise the temperature to 60°C, add 82.1g of methylglycine diacetic acid while the mixed salt solution is stirring, and keep Stirring was continued for 5 hours, the obtained solid precipitate was suction-filtered, and dried at 100°C for 3 hours to obtain 152.1 g of metal-organic framework material, 152.1 g of metal-organic framework material and 80 g of molecular sieve were mixed evenly, and kneaded with 220 g of 5% citric acid aqueous solution to form a 2.5 -3.5mm spherical particles, the spherical particles were calcined at 300° C. for 5 hours to obtain Catalyst 1 . Through ICP analysis, it is determined that in Catalyst 1, by mass, (the crystal water of the above-mentioned metal salt raw material is not counted, organic matter and carrier are not lost, and citric acid decomposition is not counted, the same below) the percentage of the following compo...
Embodiment 2
[0037] Dissolve 188.3g of cuprous nitrate and 8.9g of manganese nitrate in 1000g of distilled water to obtain a mixed salt solution, raise the temperature to 80°C, and add 125.9g of diethylenetriaminepentaacetic acid while the mixed salt solution is stirring. Maintain the temperature and continue stirring for 3 hours, filter the resulting solid precipitate with suction, and dry at 100°C for 3 hours to obtain 223.9 g of metal-organic framework material. Mix 223.9 g of metal-organic framework material with 50 g of molecular sieve and knead with 240 g of 5% citric acid aqueous solution Spherical particles of 2.5-3.5 mm were synthesized, and the spherical particles were calcined at 320° C. for 5 hours to obtain catalyst 2 . Through ICP analysis, it is determined that in catalyst 2, by mass, the following components account for the percentage of the total mass of catalyst 2: Cu34.8%, Mn1.0%, diethylenetriaminepentaacetic acid 46.0%, carrier 18.2%, through BET analysis catalyst 2 ha...
Embodiment 3
[0039] Dissolve 125.5g of cuprous nitrate and 35.3g of molybdenum nitrate in 1000g of distilled water to obtain a mixed salt solution, raise the temperature to 70°C, add 120.6g of hydroxyethylethylenediaminetriacetic acid while the mixed salt solution is stirring, and add dropwise After the end, keep the temperature and continue to stir for 5 hours, filter the obtained solid precipitate with suction, and dry at 100°C for 3 hours to obtain 193.6g of metal-organic framework material, mix 193.6g of metal-organic framework material with 60g of molecular sieve, and then use 120g of 8% citric acid The aqueous solution is kneaded into spherical particles of 2.5-3.5 mm, and the spherical particles are calcined at 300°C for 5 hours to obtain catalyst 3. Through ICP analysis, it is determined that in catalyst 3, by mass, the following components account for the percentage of the total mass of catalyst 3: Cu25.0%, Mo3.7%, 47.6% of hydroxyethylethylenediaminetriacetic acid, carrier 23.7%, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com