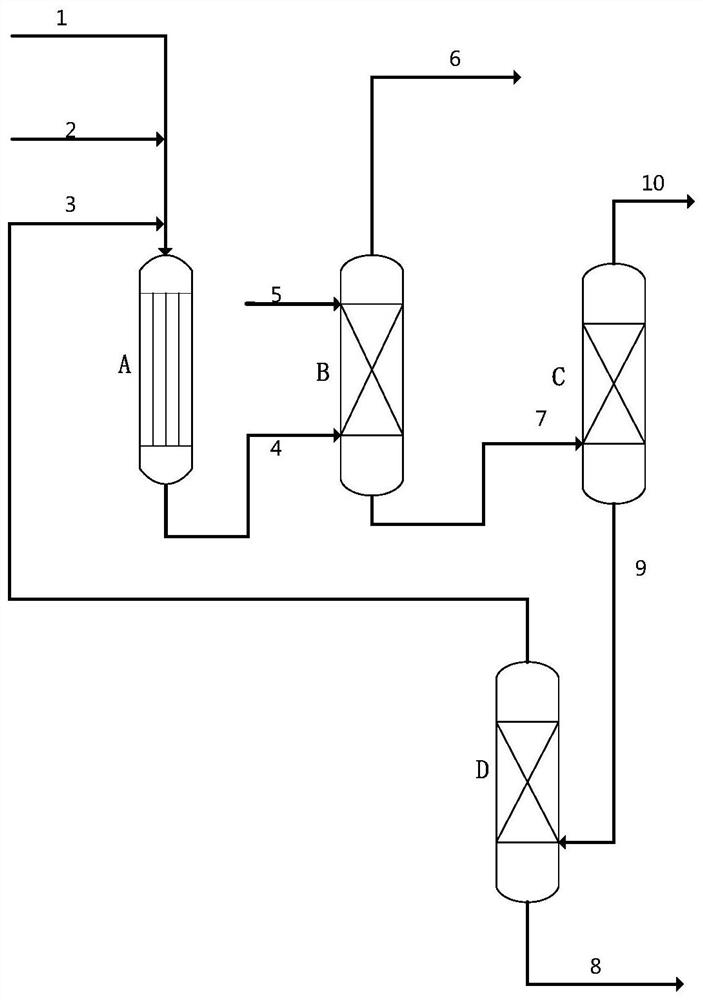
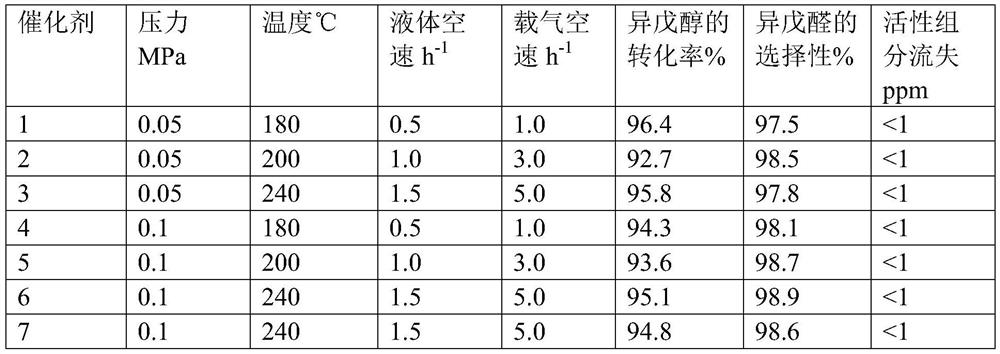
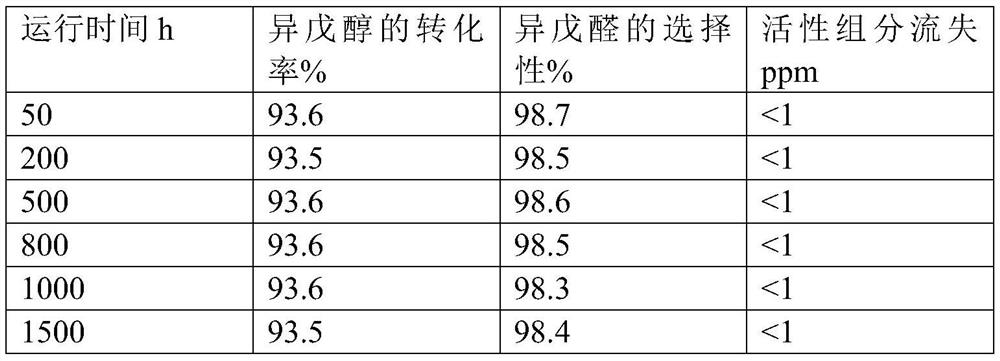
A kind of preparation method of isovaleraldehyde
A technology of isovaleraldehyde and isoamyl alcohol, applied in the chemical industry, can solve the problems of many side reactions, easy carbon deposition and deactivation of catalysts, and low production efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Dissolve 125.5g cuprous nitrate and 18.9g zinc nitrate in 1000g distilled water to obtain a mixed salt solution, heat up to 60°C, add 82.1g of methylglycine diacetic acid under the stirring state of the mixed salt solution, and maintain after the dropwise addition is completed. The temperature continued to stir for 5 hours, the obtained solid precipitate was suction filtered, and dried at 100 ° C for 3 hours to obtain 152.1 g of metal organic framework material, 152.1 g of metal organic framework material and 80 g of molecular sieve were mixed uniformly and then kneaded with 220 g of 5% citric acid aqueous solution to form 2.5 -3.5mm spherical particles, the spherical particles were calcined at 300° C. for 5 h to obtain catalyst 1. Through ICP analysis, it is determined that in catalyst 1, by mass, (the above-mentioned metal salt raw material crystal water is not counted, there is no loss of organic matter and carrier, and the decomposition of citric acid is not counted, ...
Embodiment 2
[0036] Dissolve 188.3g cuprous nitrate and 8.9g manganese nitrate in 1000g distilled water to obtain a mixed salt solution, heat up to 80°C, add 125.9g of diethylenetriaminepentaacetic acid under the stirring state of the mixed salt solution, and after the dropwise addition is completed Maintain the temperature and continue to stir for 3 hours, filter the obtained solid precipitate with suction, and dry it at 100 ° C for 3 hours to obtain 223.9 g of metal organic framework material. Mix 223.9 g of metal organic framework material with 50 g of molecular sieves and knead with 240 g of 5% citric acid aqueous solution. The spherical particles of 2.5-3.5 mm were synthesized, and the spherical particles were calcined at 320° C. for 5 h to obtain catalyst 2. It was determined by ICP analysis that in catalyst 2, by mass, the following components accounted for the percentage of the total mass of catalyst 2: Cu34.8%, Mn1.0%, diethylenetriaminepentaacetic acid 46.0%, carrier 18.2%, the ca...
Embodiment 3
[0038] Dissolve 125.5g cuprous nitrate and 35.3g molybdenum nitrate in 1000g distilled water to obtain a mixed salt solution, heat up to 70°C, add 120.6g of hydroxyethylethylenediamine triacetic acid under the stirring state of the mixed salt solution, and add dropwise. After the end, the temperature was maintained and stirring was continued for 5 hours, the obtained solid precipitate was suction filtered, and dried at 100 ° C for 3 hours to obtain 193.6 g of metal-organic framework material, 193.6 g of metal-organic framework material and 60 g of molecular sieve were mixed uniformly, and then 120 g of 8% citric acid was used. The aqueous solution was kneaded into spherical particles of 2.5-3.5 mm, and the spherical particles were calcined at 300° C. for 5 hours to obtain catalyst 3. It is determined by ICP analysis that in catalyst 3, the following components account for the percentage of the total mass of catalyst 3 in terms of mass: Cu25.0%, Mo3.7%, hydroxyethylethylenediami...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com