O-hydroxy-nitrogen silane compound and synthesis method thereof
A synthesis method and compound technology, applied in the direction of silicon organic compounds, dyed organosilicon compound treatment, organic chemical methods, etc., can solve the problems of inability to protect hydroxyl groups, restrict the universality of substrates, increase the energy consumption of synthesis processes, etc., and reach the price Inexpensive, simple and efficient synthesis method, and the effect of improving synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
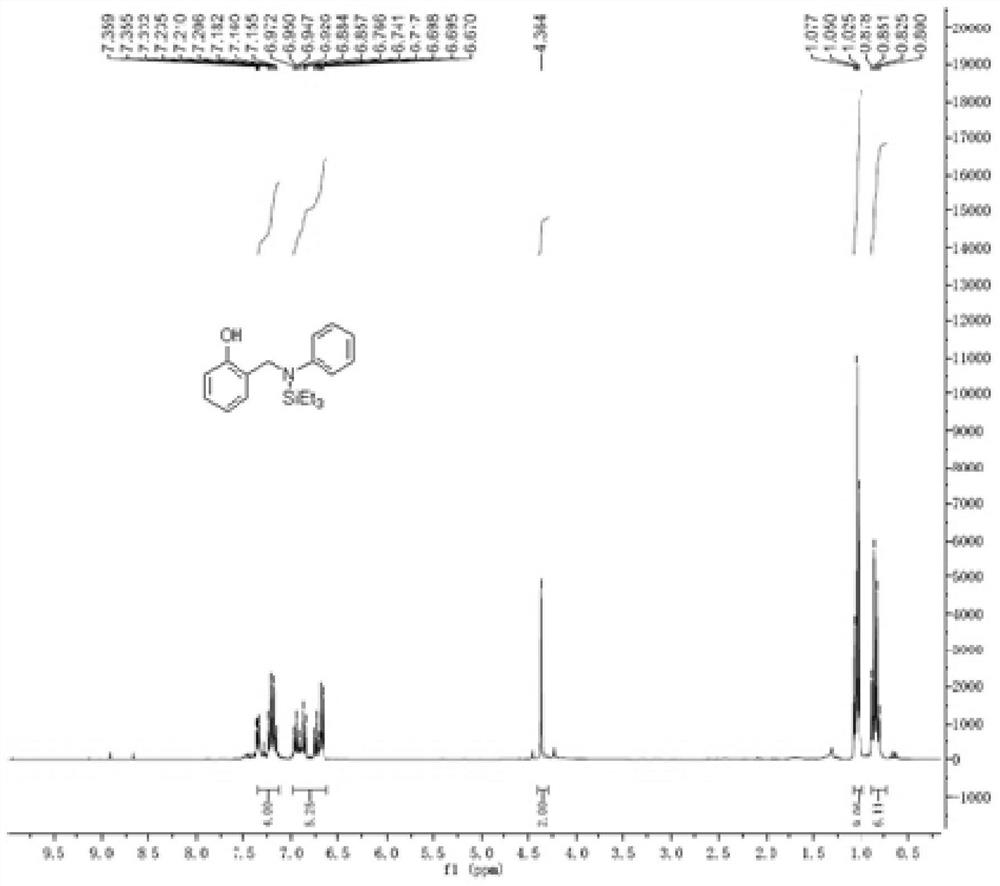
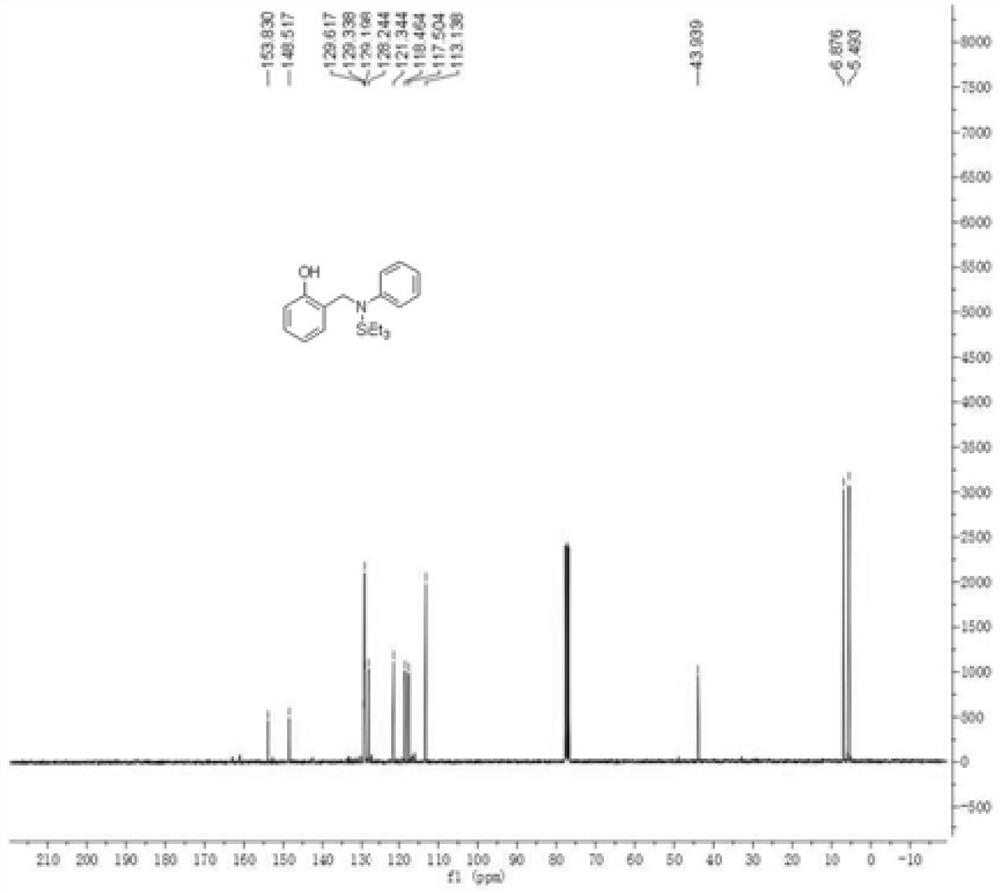
[0040] Embodiment 1: the preparation of 2-((phenyl (triethylsilyl) amino) methyl) phenol
[0041]
[0042] In a 15mL reaction tube, sequentially add salicylidene aniline (98mg, 0.5mmol), triethylsilane (116mg, 1.0mmol), tris(triphenylphosphine)carbonyl ruthenium hydrochloride (23.8mg, 0.025mmol) , under the conditions of toluene (2 mL) and nitrogen, the reaction temperature was 120 ° C under electromagnetic stirring reaction, and the reaction time was 8 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the mixture was separated by column chromatography. The eluents were ethyl acetate and petroleum ether (ethyl acetate: petroleum ether = 1:10). After separation, a yellow oil body (138 mg, 88%).
[0043] The product detection data are as follows:
[0044] 1 H NMR (300MHz, CDCl 3) δ = 7.36-7.16 (m, 4H), 6.97-6.67 (m, 5H), 4.36 (s, 2H), 1.05 (t, 9H, J = 8.1 Hz), 0.88-0.80 (m, 6H).
[0045] 13 C NMR (75MHz, CDCl 3 ) δ = 153.9, 148...
Embodiment 2
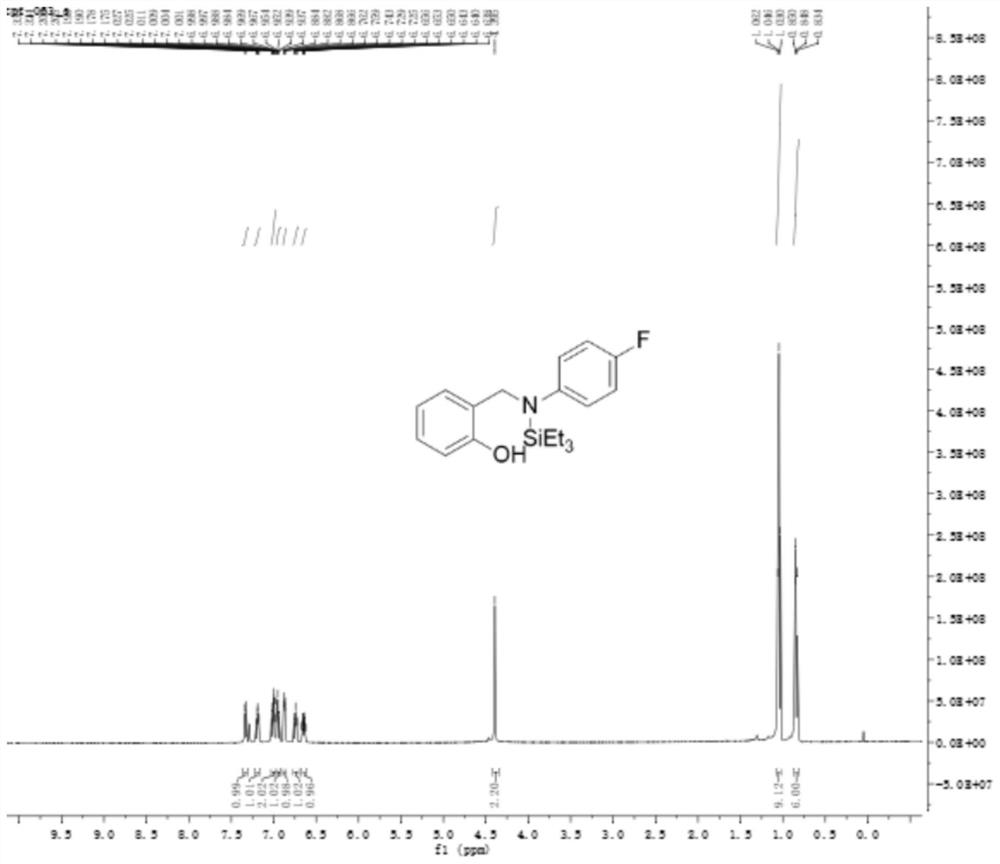
[0046] Example 2: 2-((((4-fluorophenyl)(triethylsilyl)amino)methyl)phenol
[0047]
[0048] In a 15mL reaction tube, sequentially add 4-fluoro-N-o-hydroxybenzylidene aniline (165mg, 0.5mmol), triethylsilane (116mg, 1.0mmol), tris(triphenylphosphine)carbonyl hydrochloride Ruthenium (23.8 mg, 0.025 mmol) was reacted under toluene (2 mL) and nitrogen under electromagnetic stirring at a reaction temperature of 120° C., and the reaction time was 8 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the mixture was separated by column chromatography. The eluent was ethyl acetate and petroleum ether (ethyl acetate: petroleum ether = 1:10), and a light yellow oil (85mg , 51%).
[0049] The product detection data are as follows:
[0050] 1H NMR (500MHz, CDCl3) δ=7.34-7.32(d, 1H, J=7.5Hz), 7.21-7.17(m, 1H), 7.03-6.99(m, 2H), 6.98-6.94(m, 1H), 6.88-6.76(m,1H),6.74-6.73(m,1H),6.67-6.62(m,1H),4.39(s,2H),1.06-1.03(m,9H),0.85-0.83(m,6H ).
[00...
Embodiment 3
[0052] Example 3: 2-((((4-bromophenyl)(triethylsilyl)amino)methyl)phenol
[0053]
[0054] In a 15mL reaction tube, sequentially add 4-bromo-N-o-hydroxybenzylidene aniline (137mg, 0.5mmol), triethylsilane (116mg, 1.0mmol), tris(triphenylphosphine)carbonyl hydrochloride Ruthenium (23.8 mg, 0.025 mmol) was reacted under toluene (2 mL) and nitrogen under electromagnetic stirring at a reaction temperature of 120° C., and the reaction time was 8 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the mixture was separated by column chromatography. The eluents were ethyl acetate and petroleum ether (ethyl acetate: petroleum ether = 1:10). After separation, a yellow oil body (170 mg, 87%).
[0055] The product detection data are as follows:
[0056] 1 H NMR (500MHz, CDC1 3 )δ=7.32-7.28(m,3H),7.23-7.21(t,1H,J=7.5Hz),6.98-6.95(t,1H,J=7Hz),6.90-6.89(d,1H,J=8Hz ), 6.56-6.55 (t, 2H, J=4.5Hz), 4.33 (s, 2H), 1.08-1.05 (m, 9H), 0.88-0.83 (m, 6H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com