Insoluble energetic organic polymer coated micro-nano particles and preparation method thereof
A technology of micro-nano particles and polymers, applied in transportation and packaging, boron/boride, metal processing equipment, etc., can solve the problems of harsh operating conditions, risks in scale-up tests, slow decomposition, etc., and achieve good dispersion effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of the insoluble energetic organic polymer coated micro-nano particles of the present invention comprises the following steps:
[0033] Step 1, first discuss in two cases: when the micro-nano particles are aluminum powder, add 30mL tetrahydrofuran into a 100mL three-neck flask, add 2g micro-nano aluminum powder; when the micro-nano particles are boron powder, add o-dichlorobenzene and dichlorobenzene Each 25ml of methyl acetamide is added into a 100mL three-necked flask, and 5g of micro-nano boron powder is added; micro-nano in the present invention refers to a particle size of 20 nanometers to 100 microns;
[0034] Use an ice-water bath to cool down the mixed system to 0-5°C, feed argon or nitrogen to prevent the aluminum powder from being oxidized, add the first monomer to the three-necked flask, the mass of the first monomer is 0.1% of the aluminum powder or boron powder ~20%, control the obtained system to 0~25℃;
[0035] The first monomer is...
Embodiment 1
[0044] Add 30ml of anhydrous tetrahydrofuran into a 100mL three-necked flask, add 2.0g of aluminum powder, cool down to 0°C in an ice-water bath, and inject nitrogen to prevent the aluminum powder from being oxidized. Add 184 mg of cyanuric chloride, and control the reaction solution to 20°C.
[0045] Under an ice-water bath environment at 0°C, dissolve 90 mg of ethylenediamine and 387.5 mg of acid-binding agent diisopropylamine in 5 ml of anhydrous tetrahydrofuran, and slowly drop them into the flask, which can dissolve faster than direct addition. React at 0°C for 1 hour under argon atmosphere, then gradually raise to reflux at 70°C, and react for 1 day.
[0046] The reaction solution was lowered to room temperature, and the product was obtained by suction filtration, rinsed with water, ethanol, and acetone successively, and dried in vacuum to obtain micro-nano aluminum particles coated with an insoluble energetic organic polymer.
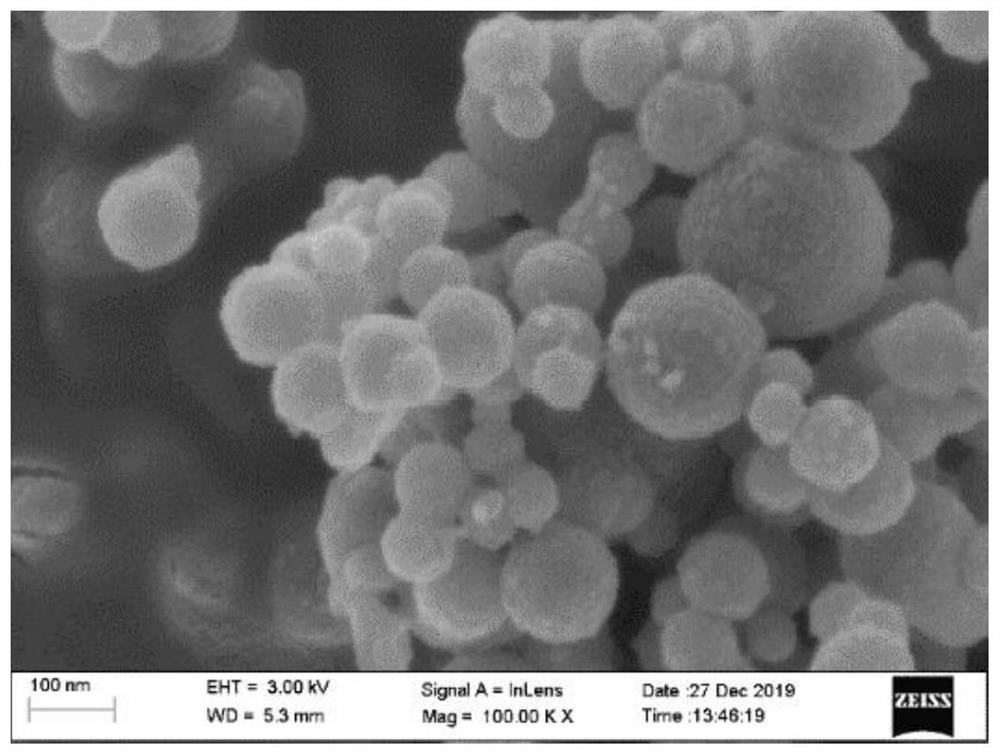
[0047] from figure 1 It can be seen that...
Embodiment 2
[0049] Add 25ml each of o-dichlorobenzene and dimethylacetamide into a 100mL three-neck flask, add 5g of boron powder, cool down to 5°C in an ice-water bath, and blow in argon to prevent the boron powder from being oxidized. Add 0.42g trialdehyde phloroglucinol, and control the reaction solution to 25°C.
[0050] In an ice-water bath environment at 3°C, slowly add 0.498g of 3,3-diaminobioxadiazole, react at 5°C for 0.5h under an argon atmosphere, then gradually rise to 120°C and react for 1 day;
[0051] After the reaction liquid is cooled to room temperature, the product is obtained by suction filtration, rinsed with water, ethanol, and acetone successively, and vacuum-dried to obtain insoluble energetic organic polymer-coated micro-nano boron particles.
[0052] from Figure 5 A large number of granular micro-nano boron particles can be seen, and there are some sheet-like polymers, and a large number of large pores are evenly distributed in the sheet-shaped polymers, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com