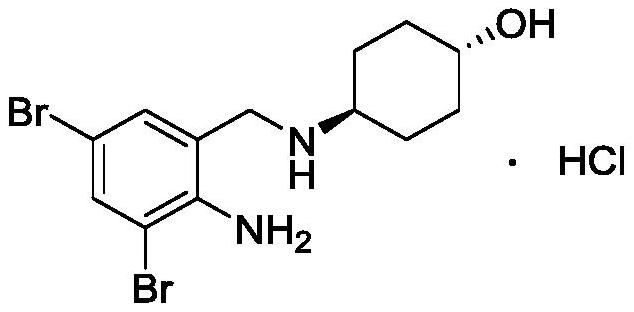
Synthetic method of ambroxol hydrochloride
A technology of ambroxol hydrochloride and a synthetic method, which is applied in the field of drug synthesis, can solve the problems of high industrial price of trifluoromethanesulfonic anhydride, high risk and difficult industrial amplification, and unfavorable cost control, etc., and achieve long-term stability in dark storage, Strong reducibility and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
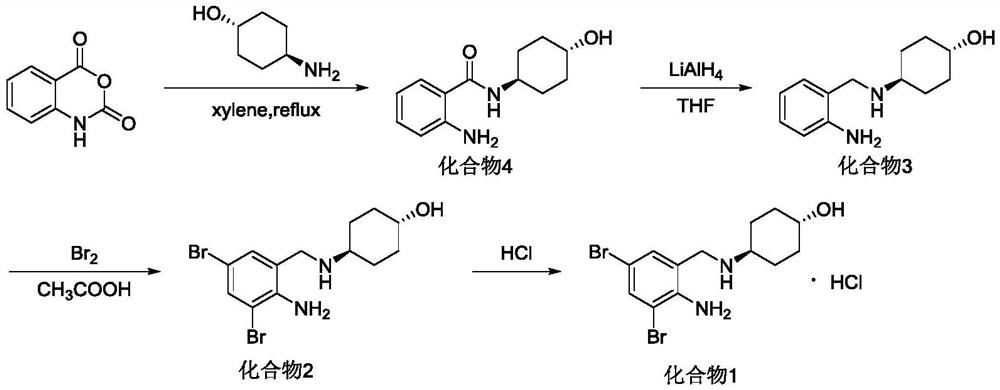
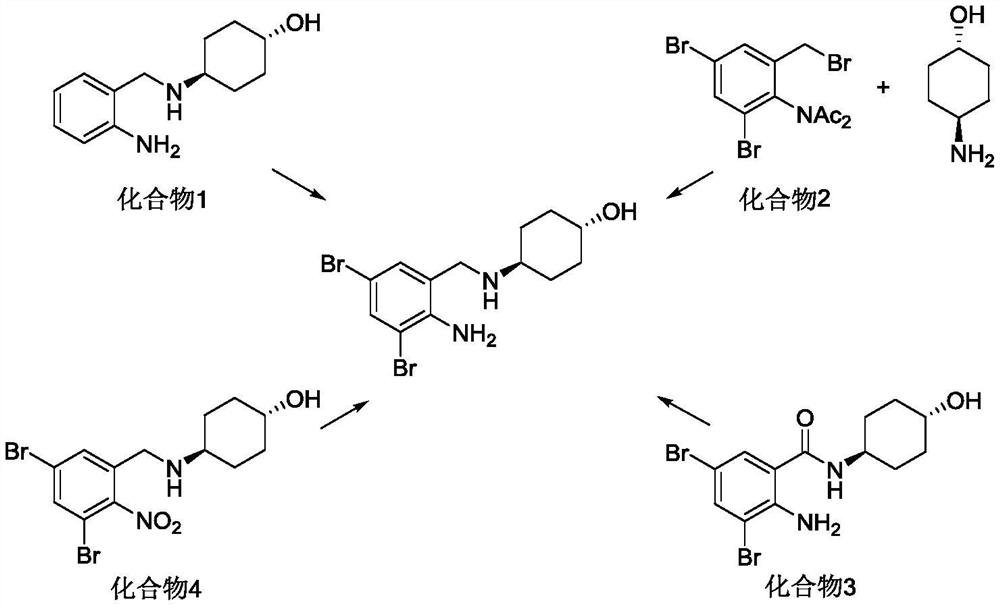
[0074] Embodiment 1: the synthesis of trans-4-[(2-aminobenzoyl) amino] cyclohexanol (compound 4)
[0075] Add 7.2g (62.5mmol) trans-4-aminocyclohexanol into a 500ml three-neck flask, add 100ml water to dissolve, add 10g (61.3mmol) isatoic anhydride in batches at 20°C, then keep warm at 30°C and stir The speed was 200 rpm, and bubbles appeared during the stirring process. After 5 hours, TLC monitoring (dichloromethane / methanol=10 / 1) showed that the reaction was complete. Suction filtration then obtains trans-4-[(2-aminobenzoyl)amino]cyclohexanol (compound 4), 10ml ice ethanol washes the filter cake twice to take away moisture, and obtains 13.5g off-white solid after drying under reduced pressure , yield 94%, mp 216°C-218°C. 1 H NMR (300MHz, DMSO-d6) δ7.92(d, J=7.7Hz, 1H), 7.50– 7.40(m, 1H), 7.18–7.07(m, 1H), 6.68(d, J=8.2Hz, 1H), 6.51(t, J=7.4Hz, 1H), 6.33(s, 2H), 4.64–4.51(m, 1H), 3.77–3.60(m, 1H), 3.41(d, J=7.0Hz, 1H ), 1.91–1.74(m,4H), 1.46–1.14(m,4H); ESI-MS, Calcd for C...
Embodiment 2
[0076] Example 2: Synthesis of trans-4-[(2-aminobenzoyl) amino]cyclohexanol (compound 4)
[0077] Add 7.45g (64.8mmol) trans-4-aminocyclohexanol into a 500ml three-neck flask, add 150ml of water to dissolve into a yellow-brown transparent liquid, add 10g (61.3mmol) isatoic anhydride in batches at 10-15°C, and react The solution turned grayish white and turbid, reacted in a water bath at 40°C, bubbles appeared when stirring continuously, and then kept warm for 2.5 hours, and the reaction was complete as monitored by TLC. Suction filtration then obtains trans-4-[(2-aminobenzoyl) amino] cyclohexanol (compound 4), washes three times with 10ml glacial ethanol and takes away moisture, obtains off-white solid 14.14g after drying under reduced pressure, yield 98.5%.
Embodiment 3
[0078] Example 3: Synthesis of trans-4-[(2-amino-3,5-dibromobenzoyl)amino]cyclohexanol (compound 3)
[0079] Add 13g (55.6mmol) trans-4-[(2-aminobenzoyl)amino]cyclohexanol (compound 4) into a 500ml three-necked flask, add 200ml of glacial acetic acid:water volume ratio=7:3 The mixture was dissolved and clarified. Add 5.7ml (111.3mmol) of bromine in 10ml of glacial acetic acid diluent dropwise under an ice bath, stir fully at a stirring speed of 200 rpm, the reaction solution turns white and turbid, and keep the temperature at 5-15°C for reaction. After the bromine drops, TLC (dichloromethane / methanol=10 / 1) monitors that the reaction is complete, add 200ml of sodium metabisulfite aqueous solution with a mass fraction of 1%, stir well, the color becomes pure white, filter with suction, filter cake with 200mL of 1% sodium metabisulfite The aqueous solution of sodium metabisulfite was stirred and neutralized, then suction filtered, washed with water, and dried to obtain 21.8 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com