Preparation method of silver-silicon catalyst, silver-silicon catalyst and application of silver-silicon catalyst
A catalyst, silver-silicon technology, applied in the field of preparation of silver-silicon catalysts, can solve the problems of high cost, unfavorable silver reduction, less lattice of Ag particles, etc. uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
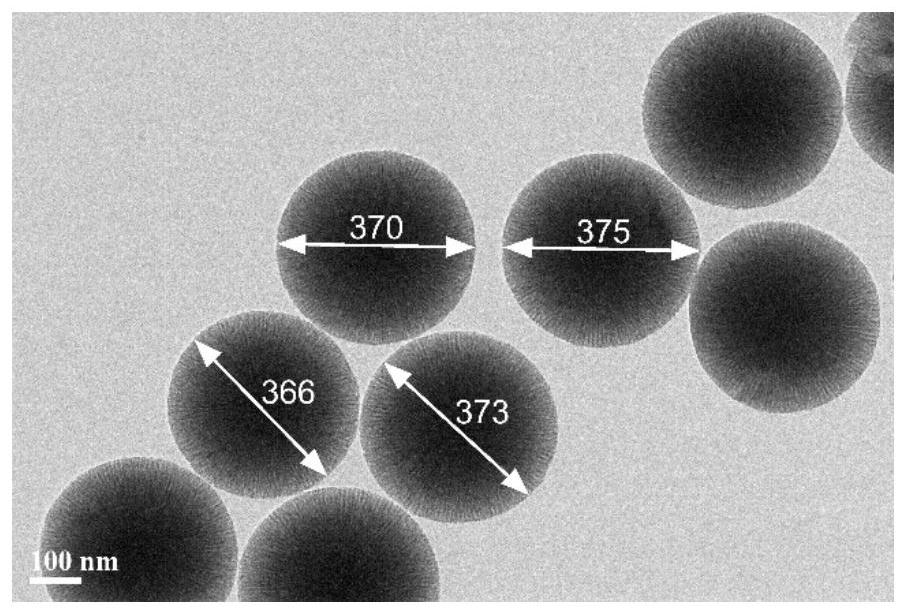
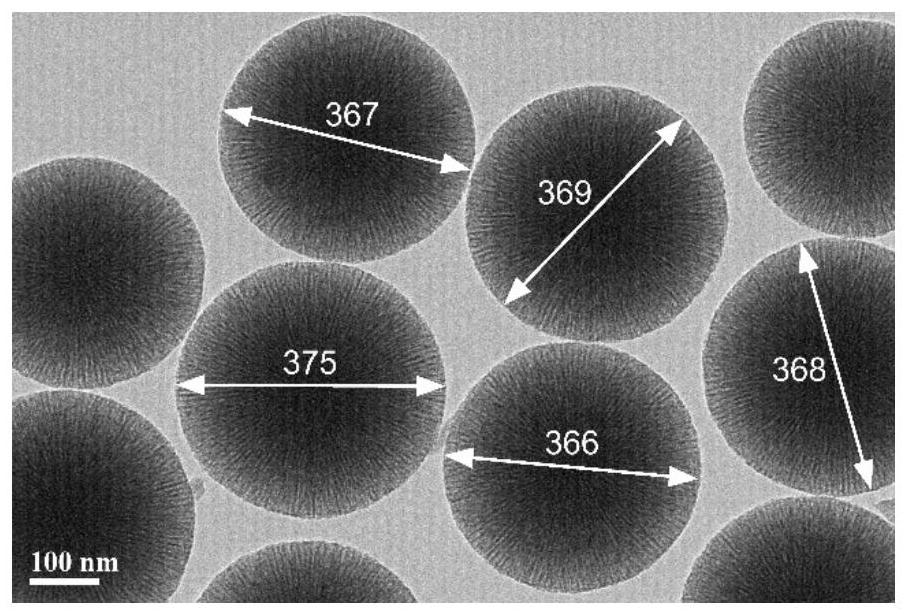
[0056] Example 1. Preparation of mesoporous silica nanospheres
[0057] The preparation of the mesoporous silica nano-microspheres of the present embodiment includes the following steps:
[0058] (1), dissolve 15g surfactant cetyl trimethyl ammonium chloride in 120ml deionized water, add 0.4g triethanolamine, be warming up to 60 ℃, under rotating speed 150r / min, stir 1 hour to obtain surface active Agent-aqueous solution as water phase; 7.5 g of tetraethoxysilane was dissolved in 25 g of cyclohexane as oil phase.
[0059] (2), the oil phase of step (1) was added dropwise to the water phase, the control temperature was 60 ° C, the rotating speed was 100 r / min, and the stirring was continued for 20 hours; after stirring, centrifugation was performed, and the sediment was washed with 120 ml of ethanol to remove residues to obtain a white solid;
[0060] (3), the white solid obtained in step (2) was dried at 120° C. for 4 hours, and then calcined at 550° C. for 4 hours to remove...
Embodiment 2
[0062] Example 2. Preparation of mesoporous silica nanospheres
[0063] The preparation method of this embodiment is basically the same as that of embodiment 1, and the difference is:
[0064] In step (1), dissolve 15g of surfactant cetyltrimethylammonium chloride in 300ml of deionized water, add 0.6g of triethanolamine as the water phase; dissolve 7.5g of tetraethoxysilane in 18.75g in cyclohexane as the oil phase.
[0065] In step (2), the temperature was controlled to be 50° C., the rotational speed was 150 r / min, and the stirring was continued for 36 hours.
[0066] The mesoporous silica nanospheres were obtained, marked as M2.
[0067] The above-mentioned M2 was observed with a transmission electron microscope, and the microscopic morphology was the same as the figure 1 similar. The diameter of M2 prepared in this example is about 490nm, and the BET specific surface area is 479m 2 g -1 , the hole volume is 1.14m 3 g -1 , with an average pore size of 9.37 nm.
Embodiment 3
[0068] Example 3. Preparation of mesoporous silica nanospheres
[0069] The preparation method of this embodiment is basically the same as that of embodiment 1, and the difference is:
[0070] In step (1), 15g of surfactant cetyltrimethylammonium chloride was dissolved in 90ml of deionized water, and 0.3g of triethanolamine was added as the water phase; 7.5g of tetraethoxysilane was dissolved in 31.25g of in cyclohexane as the oil phase.
[0071] In step (2), the temperature was controlled to be 70° C., the rotational speed was 50 r / min, and the stirring was continued for 12 hours.
[0072] The mesoporous silica nanospheres were obtained, marked as M3.
[0073] The above-mentioned M3 was observed with a transmission electron microscope, and the microscopic morphology was the same as the figure 1 similar. The diameter of M3 prepared in this example is about 220nm, and the BET specific surface area is 906m 2 g -1 , the hole volume is 0.49m 3 g -1 , with an average pore s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com