Primer for rapidly detecting exogenous virus in porcine pseudorabies live vaccine and application of primer in kit
A technology for detecting primers and porcine circovirus, which is applied in the field of molecular biology testing, can solve the problems of wasting detection time, increasing labor load, and reducing detection efficiency, and achieves easy operation, low requirements for personnel levels and laboratory conditions, and improved The effect of detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Establishment of multiplex fluorescent PCR reaction system for African swine fever virus, bovine viral diarrhea virus and porcine circovirus type 2
[0044] 1. Primer design and preparation
[0045] Find the VP72 gene sequence of different strains of African swine fever virus (ASFV), the 5'-UTR gene sequence of bovine virus diarrhea virus (BVDV) and the ORF1 gene sequence of different strains of porcine circovirus type 2 (PCV2) from Gen Bank , through the comparison analysis, select the conserved regions and design a pair of amplification primers, the sequences are as follows:
[0046] The specific primer I used for the amplification of African swine fever virus is:
[0047] SEQ ID NO. 1: upstream primer ATCCAAACAGCAGGTAAA;
[0048] SEQ ID NO.2: downstream primer CTGAGGGAATAGCAAGGT;
[0049] The specific primer II for bovine viral diarrhea virus amplification is:
[0050] SEQ ID NO.3: upstream primer GATGGCCGAATCCCTGAG;
[0051] SEQ ID NO. 4: downstream primer GTTA...
Embodiment 2
[0076] Sensitivity detection
[0077] The positive control substance in Example 1 was serially diluted 10 times, and the concentration of the standard pMD-VP72 of African swine fever virus plasmid in the mixture was 8.2×10 4 copies / μL~8.2×10 -1 copies / μL, the concentration of standard pMD-5' of bovine viral diarrhea virus plasmid is 5.7×10 4 copies / μL~5.7×10 -1 copies / μL, the concentration of porcine circovirus type 2 plasmid standard pMD-ORF1 is 3.6×10 4 copies / μL~3.6×10 -1 copies / μL, each gradient was repeated 3 times, and the multiplex fluorescence quantitative PCR detection was carried out under the guidance of the primers and TaqMan in Example 1. The detection system and detection conditions were as described in Example 1.
[0078] The test results showed that the minimum detection limit of the multiplex fluorescent PCR method for African swine fever virus was 8.2 copies / μL, the minimum detection limit for bovine viral diarrhea virus was 5.7 copies / μL, and the minimum...
Embodiment 3
[0080] specific detection
[0081] Positive control pMD-VP72 / 5' / ORF1, swine fever virus, porcine reproductive and respiratory syndrome virus, porcine pseudorabies virus, porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus, porcine rotavirus, porcine The delta coronavirus genome was used as a template to conduct a specific test, and nuclease-free water was used as a negative control; nucleic acid extraction, detection system and detection conditions were as described in Example 1.
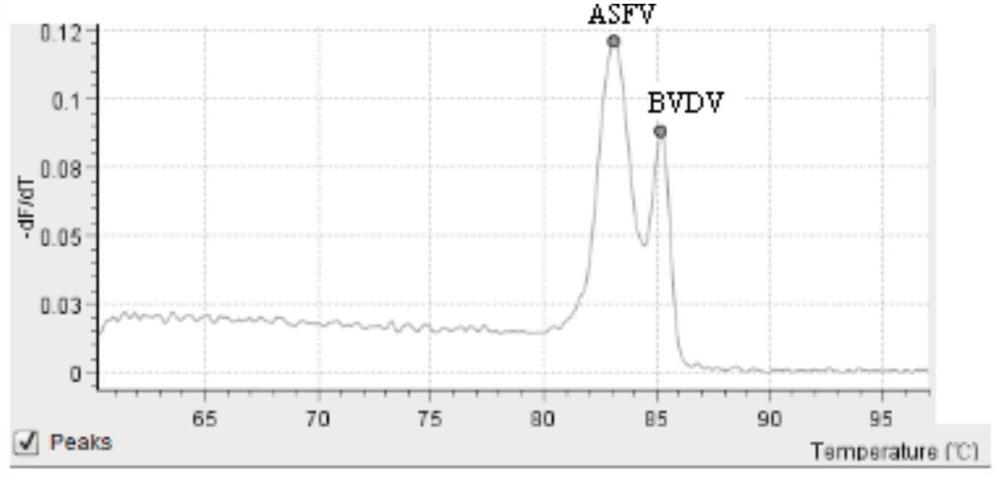
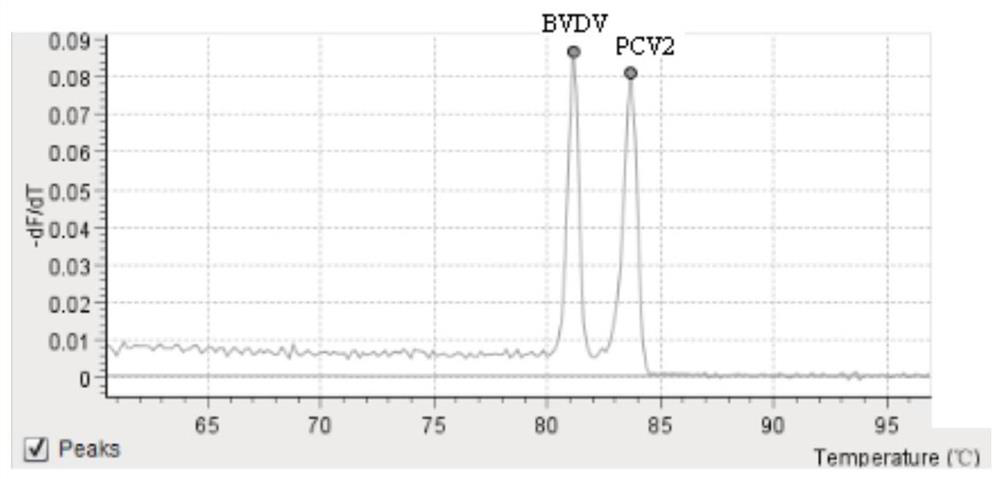
[0082] The results showed that the melting curve Tm values of the positive control pMD-VP72 / 5' / ORF1 showed melting peaks at 82±0.5°C, 84±0.5°C, and 86±0.5°C, while the other samples and negative controls did not have the above three specific peaks. It is proved that the kit of the present invention has good specificity and no crossover with other common viruses, and can specifically detect exogenous African swine fever virus, bovine viral diarrhea virus and porcine circovi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com