Preparation method of ion chelating agent containing polydopamine and obtained product
A technology of ion chelating agent and polydopamine, which is applied in chemical instruments and methods, alkali metal compounds, alkali metal oxides/hydroxides, etc., can solve the problems of little synthesis significance and a large amount of waste solvents, and achieve simple separation and operation Easy operation, convenient adsorption operation, high environmental protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
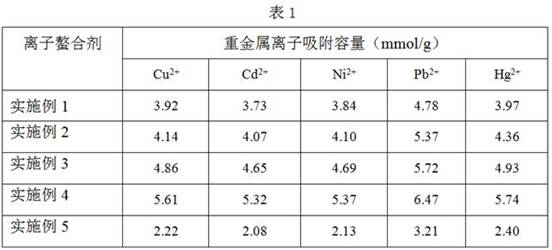
Embodiment 1
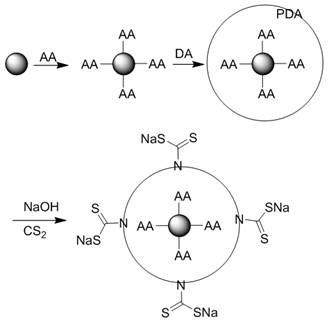
[0031] 1. Disperse 1 g of ferric oxide microspheres with a particle size of 500 nm in 100 mL of deionized water, add 0.5 g of ascorbic acid, and heat to reflux for 3 h. After the reaction, the product was separated with a strong magnet, washed three times with 20 mL of deionized water, and dried in vacuum to obtain ascorbic acid-modified Fe 3 o 4 @AA microspheres.
[0032] 2. Add 1g of the above-prepared Fe 3 o 4 @AA microspheres were uniformly dispersed in 1000 mL deionized water, 0.5 g dopamine was added, ultrasonically dispersed for 1 h, and 1000 mL PBS buffer solution (pH 7.4) was added to react for 3 h. After the reaction, the product was separated with a high-strength magnet, washed alternately with deionized water and ethanol three times (20 mL / time), and vacuum-dried to obtain Fe 3 o 4 @AAPDA microspheres.
[0033] 3. Add 1g Fe 3 o 4 @AAPDA microspheres were dispersed in 10 mL of 1 mol / L NaOH aqueous solution, and 1 g of carbon disulfide was slowly added dropwi...
Embodiment 2
[0035] 1. Evenly disperse 2 g of ferric oxide microspheres with a particle size of 100 nm in 150 mL of deionized water, add 1.5 g of ascorbic acid, and heat to reflux for 5 h. After the reaction, the product was separated with a strong magnet, washed three times with 20 mL of deionized water, and dried in vacuum to obtain ascorbic acid-modified Fe 3 o 4 @AA.
[0036] 2. Add 1 g of the above-prepared Fe 3 o 4 @AA microspheres were uniformly dispersed in 1100 mL deionized water, 1 g dopamine was added, ultrasonically dispersed for 1 h, and 1100 mL PBS buffer solution (pH 7.4) was added to react for 3 h. After the reaction, the product was separated with a high-strength magnet, washed alternately with deionized water and ethanol three times (20 mL / time), and vacuum-dried to obtain Fe 3 o 4 @AAPDA microspheres.
[0037] 3. Add 1g Fe 3 o 4 @AAPDA microspheres were dispersed in 5 mL of 2 mol / L NaOH aqueous solution, and 1 g of carbon disulfide was slowly added dropwise. Afte...
Embodiment 3
[0039] 1. Disperse 1 g of ferric oxide microspheres with a particle size of 50 nm in 100 mL of deionized water, add 1 g of ascorbic acid, and heat to reflux for 4 h. After the reaction, the product was separated with a strong magnet, washed three times with 20 mL of deionized water, and dried in vacuum to obtain ascorbic acid-modified Fe 3 o 4 @AA microspheres.
[0040] 2. Add 1g of the above-prepared Fe 3 o 4 @AA microspheres were uniformly dispersed in 1500 mL deionized water, 1 g dopamine was added, ultrasonically dispersed for 1 h, and 1500 mL PBS buffer solution (pH 7.4) was added to react for 3 h. After the reaction, the product was separated with a high-strength magnet, washed alternately with deionized water and ethanol three times (20 mL / time), and vacuum-dried to obtain Fe 3 o 4 @AAPDA microspheres.
[0041] 3. Add 1g Fe 3 o 4 @AAPDA microspheres were dispersed in 8 mL of 1 mol / L NaOH aqueous solution, and 1 g of carbon disulfide was slowly added dropwise. Af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com