Method for detecting residual quantity of morpholine in bulk drug and application of method
A detection method and a technology of raw materials, which are applied in the directions of measuring devices, material separation, and analysis of materials, etc., can solve the problems of detection of morpholine content tailing and low sensitivity, and achieve the effects of easy purchase, high sensitivity, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Determination of UV wavelength of morpholine derivatives:
[0045] Instruments and conditions: Shimadzu LC-20A liquid chromatograph (DAD detector), ZORBAX SB-C18 column, 250mm×4.6mm, 5μm, column temperature 30°C, flow rate 1.0ml per minute, injection The volume is 10 μl, the mobile phase A is water, and the mobile phase B is acetonitrile.
[0046] Using gradient elution, the elution program is shown in Table 1:
[0047] time / min Mobile phase A / % Mobile phase B / % 0-15 55 45 15-22 45 55 22-30 55 45
[0048] The specific implementation steps are as follows:
[0049] ①Preparation of derivatization reagent: just before use, weigh 158mg of 2,4-dinitrofluorobenzene, put it in a 10ml measuring bottle, dissolve it with methanol ultrasonically and dilute to the mark, shake well to get it;
[0050] ②Preparation of 2mg / ml sodium hydroxide / methanol solution: Weigh 0.1g of sodium hydroxide, put it in a 50ml volumetric flask, add methanol to ultr...
Embodiment 2
[0057] Investigation of different diluents:
[0058] Instruments and conditions: use Agilent 1260II liquid chromatograph, chromatographic column: Agilent EC-C18, 150mm×4.6mm, 4μm, detection wavelength is 374nm, column temperature is 30°C, flow rate is 1.0ml per minute, injection volume is 10μl , mobile phase A was 0.1% phosphoric acid solution, and mobile phase B was acetonitrile.
[0059] The gradient elution program is shown in Table 2.
[0060] time / min Mobile phase A / % Mobile phase B / % 0-10 60 40 10-11 45 55 11-15 25 75 15-25 60 40
[0061] The derivatization conditions are shown in Table 3.
[0062] Thinner Amount of derivatization reagent / ml Reaction temperature / ℃ Reaction time / min Acid Binder methanol, ethanol 1 60 30 sodium hydroxide
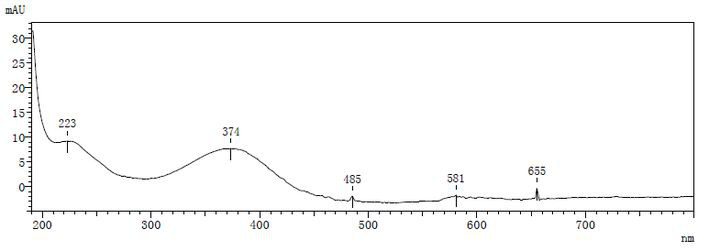
[0063] The specific implementation steps are with reference to embodiment 1, the results are as attached image 3 As shown, the derivatization reaction can be co...
Embodiment 3
[0065] Investigation of different acid-binding agents:
[0066] Apparatus and conditions: with embodiment 2.
[0067] The derivatization conditions are shown in Table 4.
[0068] Acid Binder Amount of derivatization reagent / ml Reaction temperature / ℃ Reaction time / min Thinner Sodium Hydroxide, Triethylamine 1ml 60℃ 30 minutes Methanol
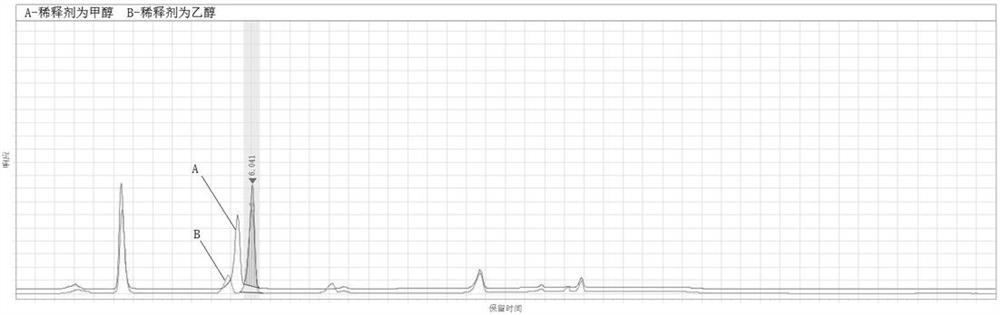
[0069] The results are attached Figure 4 As shown, the two different acid-binding agents of sodium hydroxide and triethylamine can all react completely.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com