Enzyme-sensitive polypeptide micelle type diagnosis and treatment agent and application thereof in preparation of antitumor drugs
An enzyme-sensitive, micellar technology, applied in the direction of antineoplastic drugs, drug combination, drug delivery, etc., to achieve high yield, biodegradability, functional diversification, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, the synthesis of AIEgens-1
[0052]
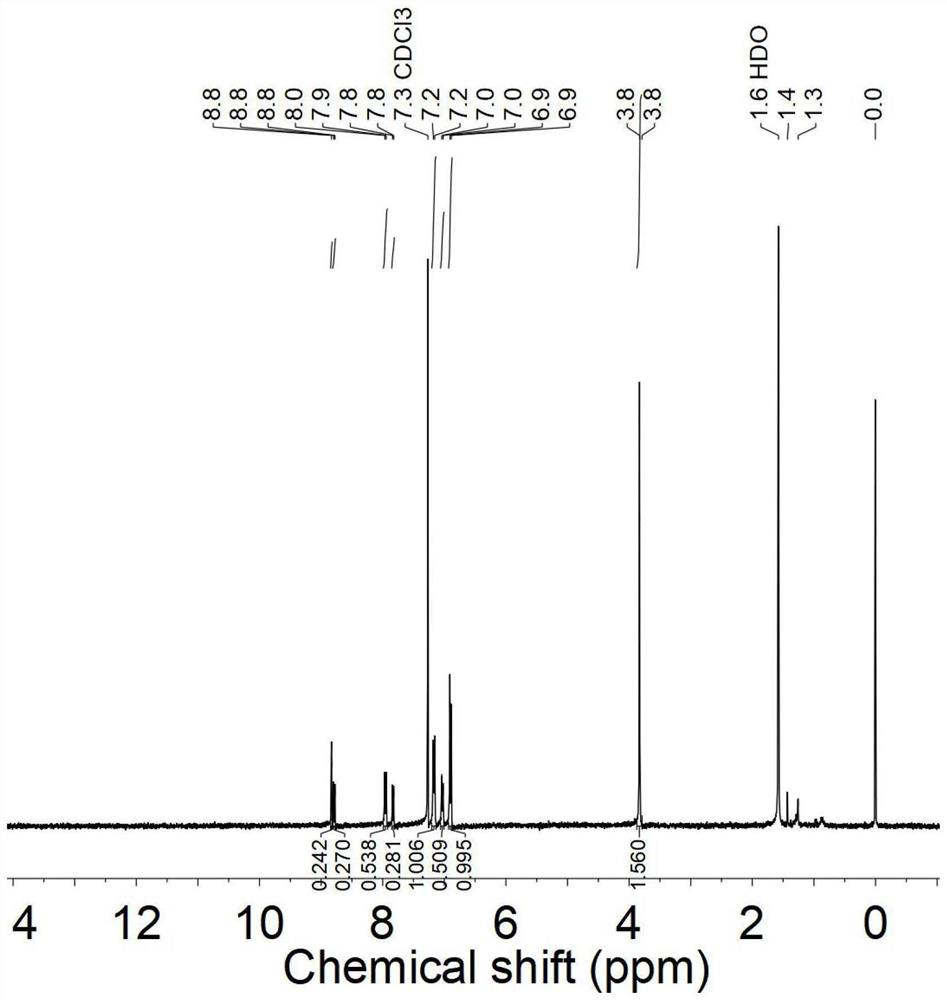
[0053] Add 1mol 4-bromo-7-(2,2-dicyanovinyl)benzo[C][1,2,5]thiadiazole, 2mol 4,4'-dimethoxy- 4 "-triphenylamine borate, 10mmol anhydrous sodium carbonate (1.06g), 0.04mmol tetrakis-(triphenylphosphine) palladium (50mg), pass into nitrogen to remove the oxygen in the flask completely. Under nitrogen atmosphere, to three-necked flask 20mL of toluene and 3mL of deionized water were added to it, and the reaction was refluxed at 110°C for 4h. After the reaction was completed, 40mL of dichloromethane and 4.0g of silica gel powder were added, and the obtained product was rotatably concentrated into a powder. Then it was placed in 60 ℃ vacuum drying oven, drying to obtain the crude product. The product is separated by silica gel column chromatography, and the mixed solvent of dichloromethane / petroleum ether with a volume ratio of 4:1 is used as the eluent, and finally purified and dried to obtain purple Black solid (yield:...
Embodiment 2
[0054] Embodiment two, amphiphilic small molecule peptide (FMOC 2 -K-GFLGG-R 8 Synthesis of GD)
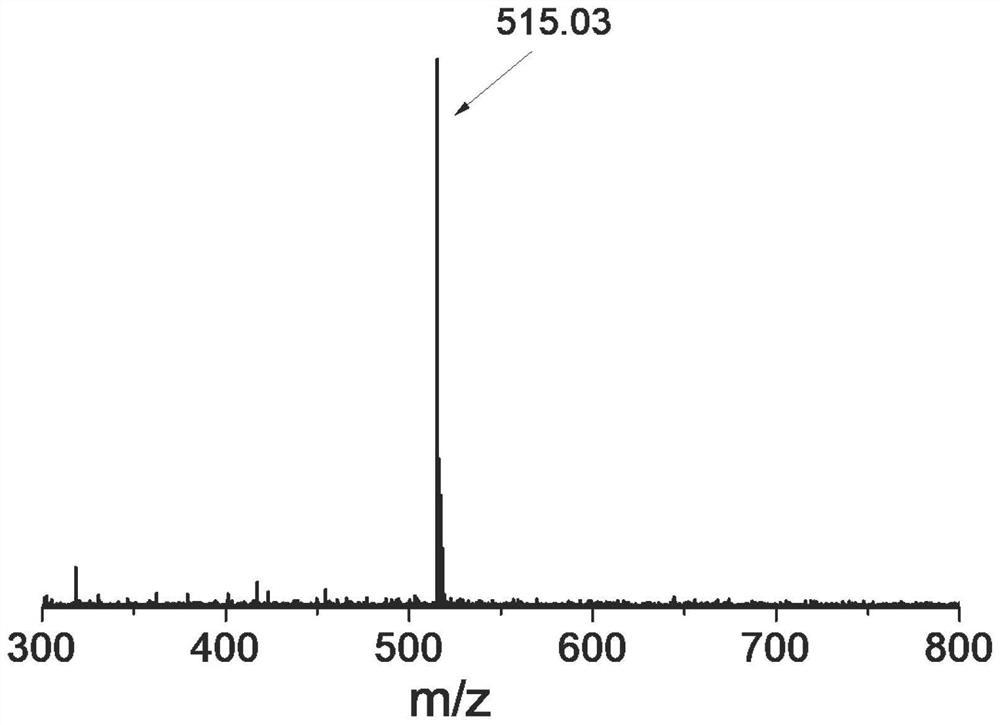
[0055] FMOC was synthesized by standard Fmoc solid-phase peptide synthesis method 2 -K-GFLGG-R 8GD: Weigh 2-chloro-trityl chloride resin (0.8g, 0.97mmol / g) into the peptide solid-phase synthesis column, add 20mL N,N-dimethylformamide (DMF) to soak the resin for 30 minutes, and make After it was fully swollen, the DMF was removed by suction filtration. Prepare to insert the first amino acid and enter the amino acid condensation reaction. Then, a DMF solution in which Fmoc-Asp(OtBu)-OH (3 times the degree of substitution of the resin) and DIEA (6 equivalents of the degree of substitution of the resin) was dissolved was added to the polypeptide solid-phase synthesis column, and reacted for 2 hours. After the reaction, the filtrate was sucked away and washed 4 times with DMF. The peptide resin after condensation of the first amino acid protected by FMOC was obtained. Then add 2...
Embodiment 3
[0057] Embodiment three, the synthesis of AIEgens-1@MPA
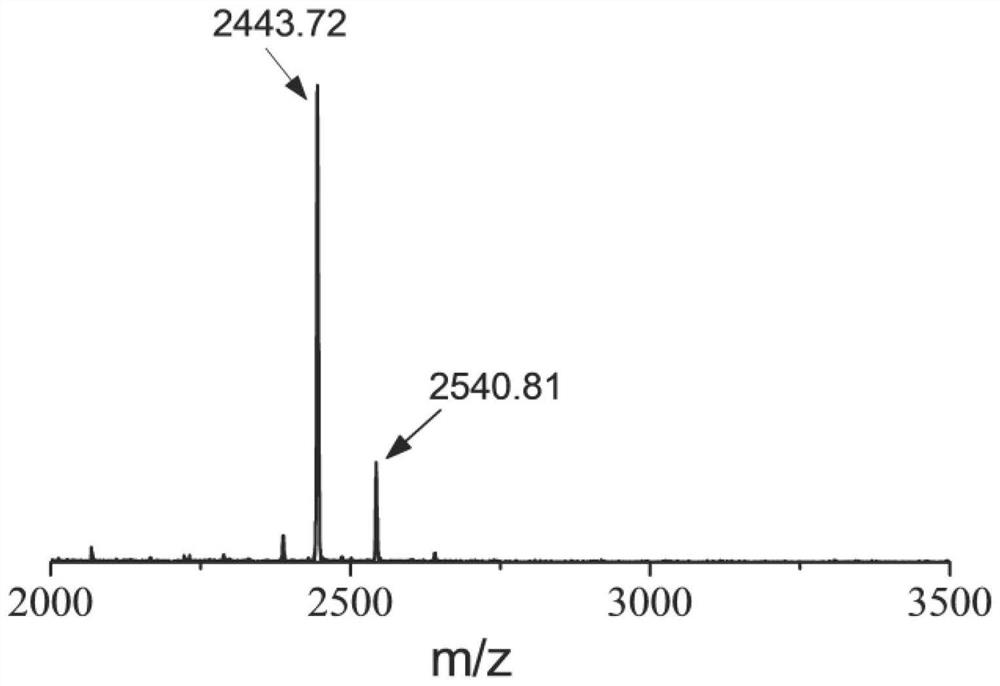
[0058] 50mg AIEgens-1 (prepared in Example 1), 50mg FMOC 2 -K-GFLGG-R 8 GD (prepared in Example 2) and 20 mL of DMF were added into a single-necked round-bottomed flask, and the reaction was stirred at room temperature for 24 h. Subsequently, the above solution was added to a dialysis bag (MWCO 1000Da), dialyzed in ultrapure water for 24 hours, and the ultrapure water was changed every 4 hours. After the dialysis, the aqueous solution in the dialysis bag is frozen into a solid, and then placed in a lyophilizer to freeze-dry into a powder. Characterization of FMOC by Transmission Electron Microscopy (TEM) 2 -K-GFLGG-R 8 The morphology and size of micelles formed by self-assembly of GD small molecule peptides, such as Figure 5 shown. from Figure 5 It was observed in TEM that the micelles are spherical and have good dispersion. The dry state particle size of the micelles is 35nm±1nm, which proves that the amphiphi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com