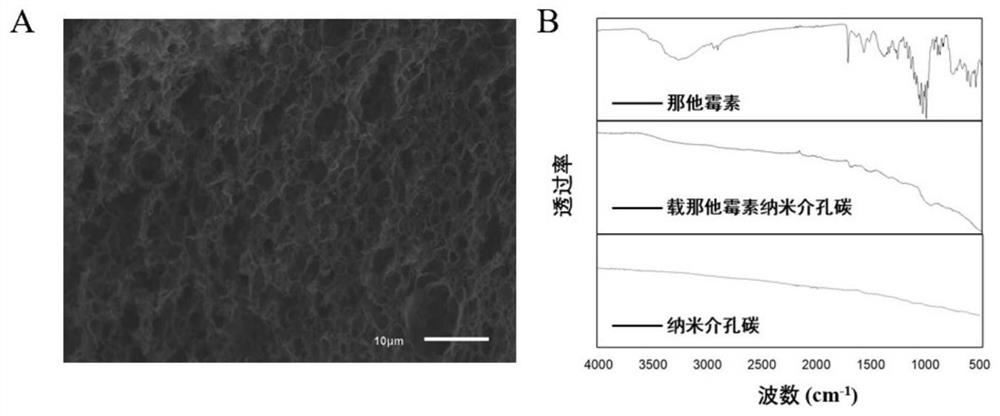
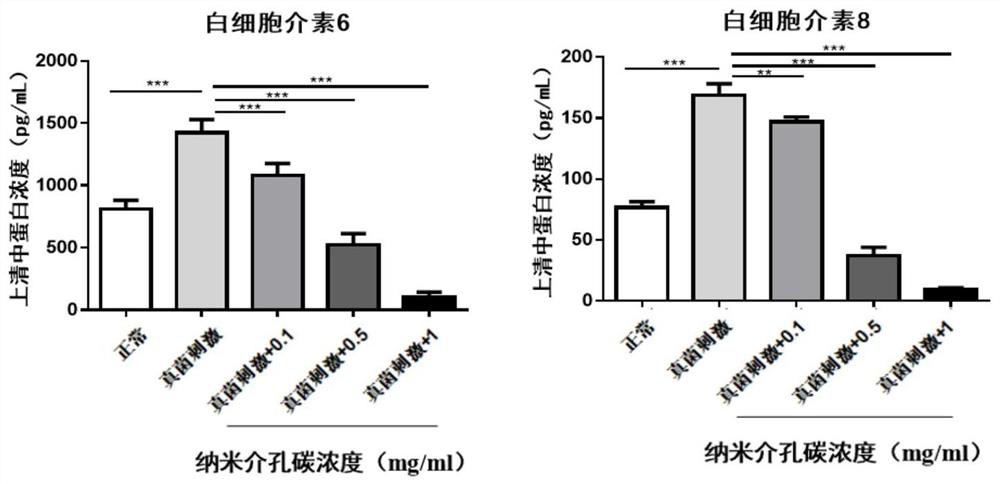
Nanometer mesoporous carbon eye drops loaded with natamycin and silver as well as preparation method and application thereof
A nano-mesoporous and natamycin technology, applied in nano-carbon, nano-technology for materials and surface science, nano-technology, etc., can solve the high blinding rate of fungal keratitis and ocular surface residence time Short, strong irritation and other problems, to achieve the effect of improving solubility and bioavailability, prolonging antifungal time, and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This embodiment relates to the preparation method of nano-mesoporous carbon eye drops loaded with natamycin and silver, and the specific steps are as follows:
[0035] 1) Dissolve 5g of sodium alginate in 500mL of double-distilled water, stir at room temperature for 4 hours, configure a sodium alginate solution with a mass volume concentration of 10mg / mL, and drop it into 500mL of calcium chloride with a concentration of 0.1mol / L solution, stirred at room temperature for 8 hours, and filtered through gauze to obtain calcium alginate gel;
[0036] 2) Put the calcium alginate gel in a freeze dryer (-60°C) for 48 hours, then put it into a tube furnace, heat it to 950°C in a nitrogen atmosphere (the heating rate is 5°C / min), and keep it warm for 1 Hour;
[0037] 3) Place the carbonized sample in a hydrochloric acid solution with a molar concentration of 1 mol / L, soak it overnight at room temperature, then filter and wash it with double distilled water and ethanol, and plac...
Embodiment 2
[0051] This embodiment relates to the preparation method of nano-mesoporous carbon eye drops loaded with natamycin and silver, and the specific steps are as follows:
[0052] 1) Dissolve 2.5g of sodium alginate in 500mL of double-distilled water, stir at room temperature for 4 hours, configure a sodium alginate solution with a density of 5mg / mL, and drop it into 500mL of calcium chloride with a molar concentration of 0.1mol / L In the aqueous solution, stir at room temperature for 8 hours, and obtain calcium alginate gel after filtering through gauze;
[0053] 2) Put the calcium alginate gel in a freeze dryer (-60°C) for 48 hours, then put it into a tube furnace, heat it to 950°C in a nitrogen atmosphere (the heating rate is 5°C / min), and keep it warm for 1 Hour;
[0054] 3) Place the carbonized sample in 1mol / L aqueous hydrochloric acid solution, soak overnight at room temperature, filter and wash with double distilled water and ethanol, and place it in a blast dryer (60°C) fo...
Embodiment 3
[0059] This embodiment relates to the drug release ability and influencing factors analysis experiment of nano mesoporous carbon loaded with natamycin, specifically:
[0060] (1) In vitro drug release experiment
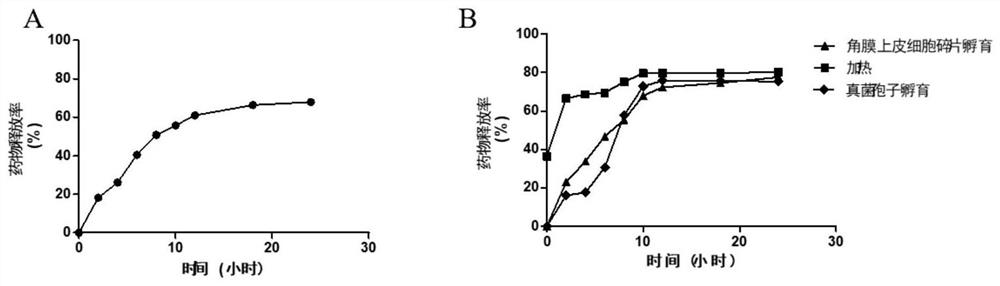
[0061] Weigh 2 mg of natamycin-loaded nano-mesoporous carbon, add 3 mL of PBS solution, put it into a dialysis bag, clamp both ends, and put it into a beaker with 50 mL of PBS solution at a temperature of 37 ° C and a rotation speed of 100 r / min, take out 2mL of dialysate every 2 hours, and immediately replenish the same amount of PBS solution, measure the absorbance of the dialysate at 304nm, calculate its concentration according to the regression equation, and calculate the cumulative drug release rate: cumulative drug release rate = Released drug amount / administered amount × 100%. The result is as figure 2 As shown in A.
[0062] From figure 2 It can be seen from A that the release of natamycin presents sustained release, with a release rate of about 56% in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com