Zinc hydrometallurgy process adopting chloride system
A technology for hydrometallurgy of zinc and chloride, which is applied in the direction of photography technology, photography auxiliary technology, process efficiency improvement, etc., can solve the problems of low chloride ion tolerance, easy decomposition, low solubility, etc., and achieve good economic benefits and Market prospect, easy large-scale production, and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
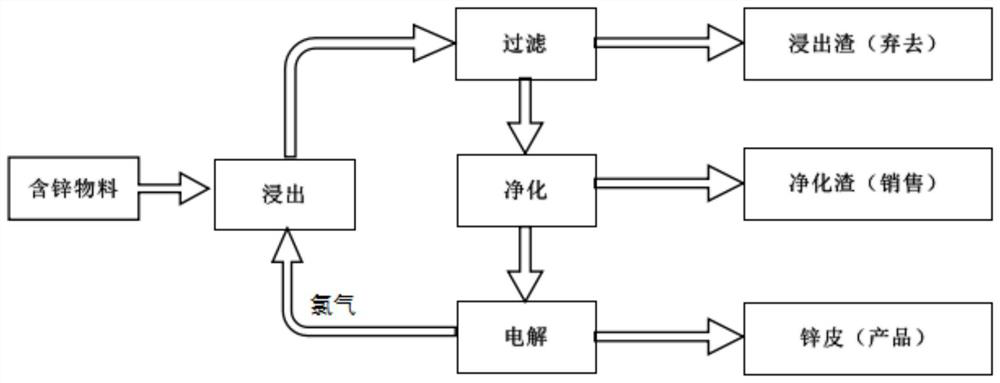
[0056] Therefore, in view of the defects of the above-mentioned method, the present invention provides a kind of hydrometallurgy process of chloride system, the flow process of this process is as follows figure 1 , the reaction process can be briefly expressed as:
[0057] Zinc oxide leaching: Cl 2 +H 2 O=HCl+HClO
[0058] 2HClO=2HCl+O 2 (Decomposition of hypochlorous acid by light or catalyst)
[0059] 2HCl+ZnO=ZnCl 2 +H 2 o
[0060] Zinc sulfide leaching: ZnS+Cl 2 =ZnCl 2 +S
[0061] Electrolysis: ZnCl 2 =Zn+Cl 2
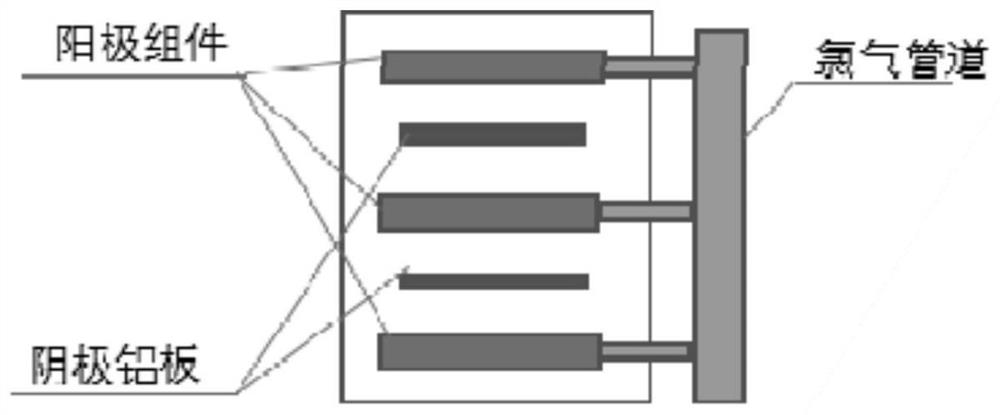
[0062] This process uses the chlorine gas produced by the anode during electrolysis to leach zinc oxide and zinc sulfide raw materials, and obtain an aqueous solution with zinc chloride as the main component after leaching. Purify the aqueous solution to remove impurities such as lead, copper, indium, etc. to obtain zinc chloride electrolyte. Pump the electrolyte solution into the electrolytic cell, turn on the electricity for electrolysis, the chlor...
Embodiment 1
[0089] The zinc-containing raw material is recycled zinc raw material, and the recycled zinc raw material is ground to a fineness of 200-400 mesh, and the test data are as follows:
[0090] Zn Cl S Fe Cu Pb Cd 51.7% 12.2% 0.13% 5.2% 0.04% 3.7% 0.29%
[0091] Zn Cl Fe Cu Pb+Cd K+Na pH 29g / L 116g / L 3.88mg / L - 2.2mg / L 77.3g / L 4.8
[0092] In addition, the composition data of electrolytic waste liquid are as follows:
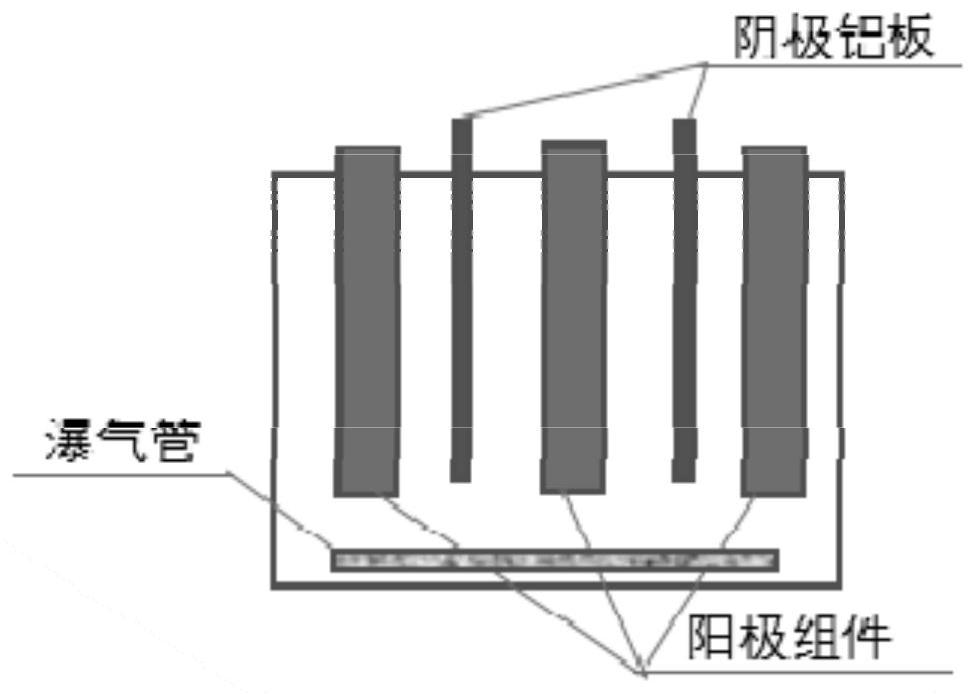
[0093] Take 3.5Kg of the above-mentioned solid powder raw material, mix it with 14L electrolytic waste liquid, stir it into a uniform slurry, pump it into a packed absorption tower with a special structure at a speed of 2.5L / min, and feed chlorine gas (containing air , chlorine purity ≈55%), and the feeding rate is about 10L / min. For the specific structure of the packed absorption tower and the specific implementation process of chlorine feeding, please refer to the Chinese invention patent "...
Embodiment 2
[0109] The zinc-containing raw material is zinc sulfide raw material, and the zinc sulfide raw material is ground to a fineness of 200-400 mesh, and the test data are as follows:
[0110] element S Zn Pb Cu Si Fe content% 26.2 50.8 2.06 0.017 4.7 5.8
[0111] Zn Cl Fe Cu Pb+Cd K+Na pH 31g / L 133.7g / L 10.5mg / L - 0.8mg / L 86.6g / L 5.4
[0112] In addition, the composition data of electrolytic waste liquid are as follows:
[0113] Take 3.5Kg of the above-mentioned solid powder raw material, mix it with 17L electrolytic waste liquid, stir it into a uniform slurry, pump it into the packed absorption tower at a speed of 2.5L / min, and feed chlorine gas (containing air, chlorine gas purity ≈55 %), the feed rate is about 10L / min.
[0114] During the aeration process, check the pH of the liquid every half hour, and keep the pH of the liquid between 3.5 and 5.0 by adding sodium hydroxide or hydrochloric acid solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com