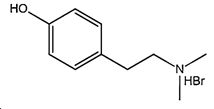
Preparation method of hordenine hydrochloride
A technology of hordenine hydrochloride and maltine hydrobromide, which is applied in the field of preparation of hordenine hydrochloride, and can solve the problems of complicated preparation process, increased operation difficulty, high price of sodium triacetoxyborohydride, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
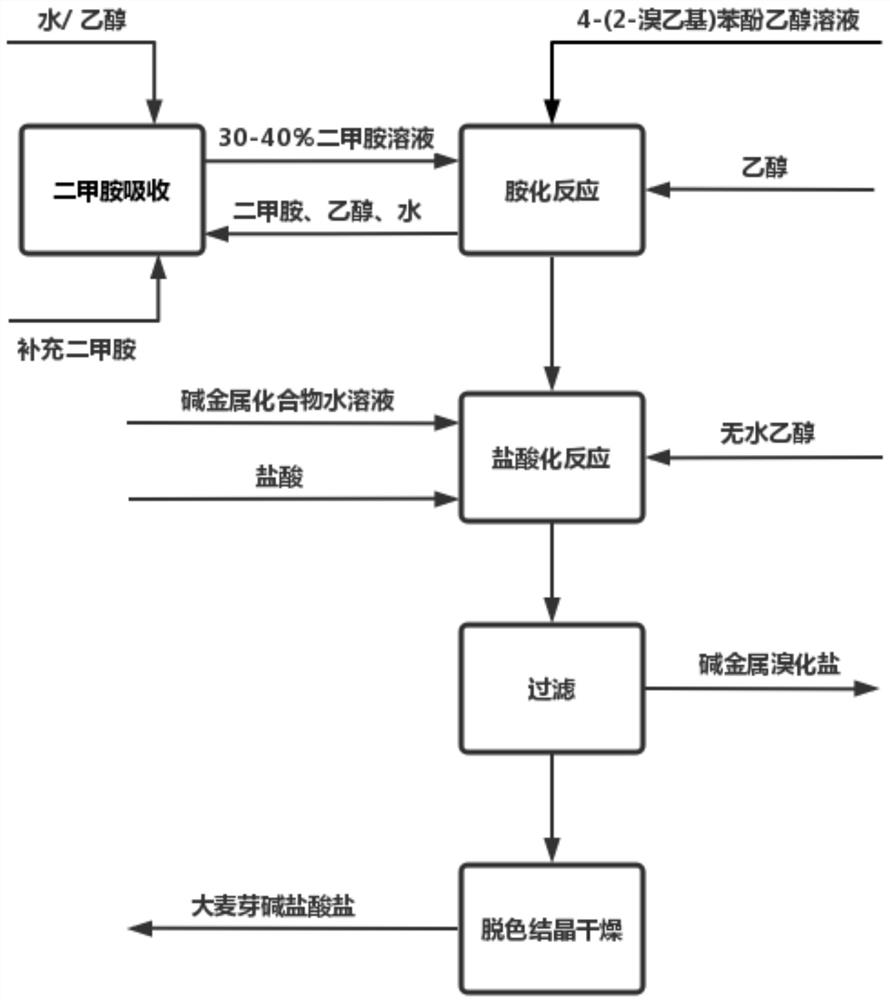
[0095] Preparation of hordenine hydrobromide:
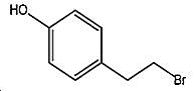
[0096] Take 40% (weight) of 1970 grams of dimethylamine absolute ethanol solution industrial product (including 17.5mol of dimethylamine) and add it to a 5-liter capacity reflux ice brine condenser, thermometer, dripping bottle, stirrer In a round four-necked glass reaction flask, the reaction flask was placed in an ice-water bath; the temperature was lowered to 0°C with stirring, and 610 g of 4-(2-bromoethyl)phenol (content 99%, 2.5 mol) and 1420 g of absolute ethanol; keep the temperature of the reaction solution at 0-5°C during the dropwise addition. After the dropwise addition, the ice-water bath was removed, and the reaction was stirred at room temperature for 12 hours; then, it was changed to a water bath and heated to 75°C, and the excess dimethylamine gas was distilled for the first time. Water ethanol absorbs as absorbing liquid; Distill out ethanol again to crystallization occurs for the second time, then de-dry in vac...
Embodiment 2
[0099] Preparation of hordenine hydrobromide:
[0100] By the operation process of embodiment 1; Difference is: use 1970 grams of dimethylamine aqueous solution industrial products of 40% (weight); After dropwise addition, water bath is warmed to 15 ℃, and control reaction temperature is 15~20 ℃ Stir the reaction for 18 hours; then, change to a water bath and heat to 75°C, distill off excess dimethylamine gas for the first time, and use the ethanol mixture containing low-concentration dimethylamine distilled in Example 1 for the second time as The absorption liquid of dimethylamine gas; Distill out ethanol again to crystallization occurs for the second time, obtain 582 grams of light yellow hordenine hydrobromide, the yield is 94.5% in terms of 4-(2-bromoethyl)phenol .
[0101] Wherein, the first distillation absorption obtains 2200 grams of ethanol and water mixed solutions containing low-concentration dimethylamine, wherein the dimethylamine content is 30% (weight); the sec...
Embodiment 3
[0103] Preparation of hordenine hydrobromide:
[0104] Add 3500ml of 40% dimethylamine aqueous solution industrial product (3150g, containing 28mol of dimethylamine) into a 5000ml round four-necked glass reaction flask with reflux ice-salt water condenser, thermometer, dropping bottle and stirrer , the reaction bottle was placed in an ice-water bath; stirred and cooled to 0 ° C, slowly added dropwise through a dropping bottle made of 187 grams of 4-(2-bromoethyl) phenol (content 99%, 0.93mol) and 1240 grams of absolute ethanol The mixed solution formed; keep the temperature of the reaction solution at 0-5°C during the dropwise addition process; remove the ice water bath after the dropwise addition, and heat the water bath to 25°C after the dropwise addition, and control the reaction temperature at 25°C to 30°C The reaction was stirred at room temperature for 12 hours. Then, change to a water bath and heat to 90°C for the first time to distill excess dimethylamine (use the wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com