Method for preparing nanoscale lithium ferric manganese phosphate material by using co-crystallization method
A nano-scale technology of lithium iron manganese phosphate, which is applied in the preparation of intermediates of lithium iron manganese phosphate, and in the field of preparation of nanoscale lithium iron manganese phosphate materials, can solve the problems of limited gram capacity, large primary particle size, and uneven distribution. Achieve good shape, good stability, and ensure consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
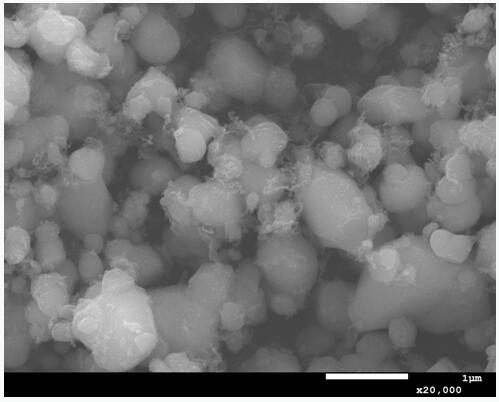
[0025] Dissolve manganese sulfate, ferric sulfate, and calcium dihydrogen phosphate in 3L of pure water at a molar ratio of 0.8:0.2:1, stir for 1 hour, add calcium hydroxide to adjust the pH to 2.0, continue stirring for 2 hours to form a mixed slurry, and filter the mixed slurry , take the clear liquid and add it to the oxidation kettle, add hydrogen peroxide to fully stir the reaction, stir the reaction for 1-3h, and the reaction temperature is 25-120°C, after the reaction press filter, wash, and remove impurities to obtain initially pure nano-iron manganese phosphate, and then Dry at 50°C for 8 hours to obtain Mn 0.8 Fe 0.2 PO 4 intermediate, see attached figure 1 .
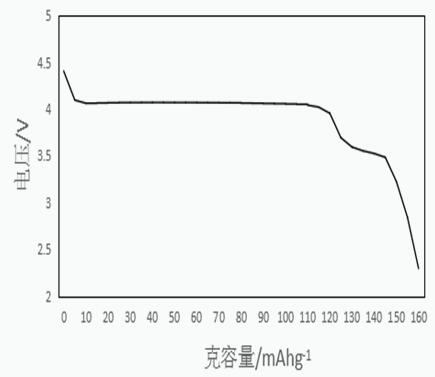
[0026] Feed this intermediate with lithium carbonate and sucrose at a ratio of 1:1.01:0.3, dissolve in pure water, and then sand mill in a sand mill for 2 hours, and dry the slurry at 50°C at a temperature of 30-80°C. Dry for 6h, then in N 2 Under the protection of the atmosphere, calcined at 700°C for 12...
Embodiment 2
[0030] Dissolve manganese nitrate, ferric nitrate, and ammonium dihydrogen phosphate in 3L of pure water at a molar ratio of 0.7:0.3:1, stir for 1 hour, add sodium hydroxide to adjust the pH to 3.0, continue stirring for 2 hours, add hydrogen peroxide to stir the reaction, Stir and react for 1-3h, the reaction temperature is 25-120°C, press filter, wash, and remove impurities after full reaction to obtain initially pure nano-iron manganese phosphate, and then dry at 80°C for 5h to obtain Mn 0.7 Fe 0.3 PO 4 Intermediate;
[0031] Feed this intermediate with lithium carbonate and PEG according to the ratio of 1:1.02:0.2, dissolve in pure water, and then sand mill in a sand mill for 2 hours, dry the slurry at 80°C for 4 hours, and then under the protection of N2 atmosphere , calcined at 650°C for 12h, the heating rate is 5°C / min, and finally cooled to room temperature naturally, and crushed with a pulverizer to obtain a product with a particle size of 50-400nm, which is LiMn 0...
Embodiment 3
[0034] Dissolve manganese acetate, ferric oxalate, and ammonium dihydrogen phosphate in 3L of pure water at a molar ratio of 0.6:0.4:1, stir for 1 hour, add ammonia water to adjust the pH to 2.5, continue stirring for 2 hours, add hydrogen peroxide and stir to react, stir to react 1-3h, the reaction temperature is 25-120°C, after the reaction press filter, wash, and remove impurities to obtain initially pure nano-iron manganese phosphate, and then dry at 60°C for 6h to obtain Mn 0.6 Fe 0.4 PO 4 Intermediate;
[0035] Feed this intermediate with lithium carbonate and acetylene black at a ratio of 1:1.01:0.1, dissolve in pure water, and then sand-mill in a sand mill for 2 hours, dry the slurry at 70°C for 5 hours, and then protect it under N2 atmosphere Calcined at 650°C for 15 hours, with a heating rate of 5°C / min, and finally cooled to room temperature naturally, and crushed with a pulverizer to obtain a product with a particle size of 50-500nm, which is LiMn 0.6 Fe 0.4 PO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com