Novel fluorescent probe for simultaneously and quantitatively detecting Cys, Hcy and GSH in plasma as well as preparation method and application of novel fluorescent probe
A fluorescent probe and quantitative detection technology, applied in the field of fluorescence detection, can solve the problems of few reports on fluorescent probes, achieve the effects of reducing impurities that are difficult to separate, simple synthesis method, and preventing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
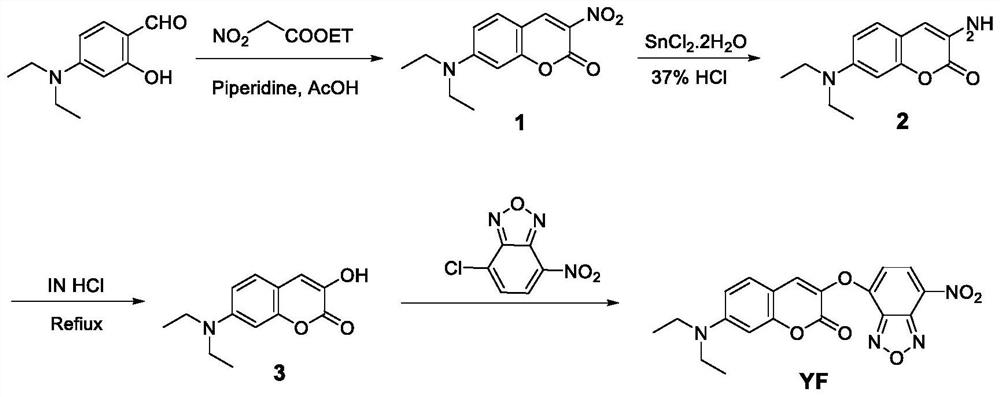
[0040] The synthesis of fluorescent probes, the specific steps are as follows:
[0041] (1) Add 1.1 g of 4-(diethylamino) salicylaldehyde to a 50 mL flask, dissolve it with 13 mL of n-butanol, then add 0.6 mL of ethyl nitroacetate, stir well, then add 74 μL of piperidine, and then Add 0.26mL of acetic acid, heat the mixture under reflux at 102°C and stir for 11 hours; after the reaction, cool to room temperature, filter the precipitate with suction to obtain a filter cake, wash and purify the precipitate with n-butanol and petroleum ether, and dry in vacuo to obtain 7- (Diethylamino)coumarin-3-nitro orange solid 1.1g, yield 81%.
[0042] (2) Add 3mL of hydrochloric acid solution to a 50mL flask, then add 0.65g of stannous chloride in an ice-bath environment, and then add 0.21g of 7-(diethylamino)coumarin-3-nitro , stirred at room temperature, reacted for 1.5h, added sodium hydroxide to neutralize the pH to 7 under an ice bath, extracted and separated the mixed solution with d...
Embodiment 2
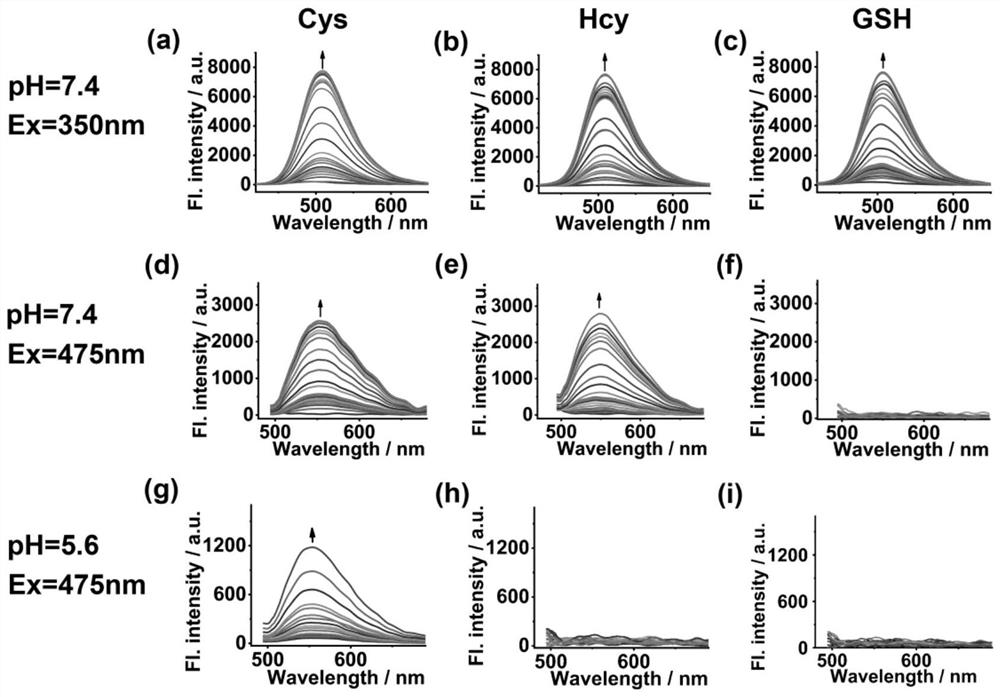
[0046] Fluorescent spectral responses of the fluorescent probes obtained in Example 1 to the fluorescent detection of Cys, Hcy and GSH.
[0047] Prepare a 5 μM fluorescent probe test solution with 20 mM potassium phosphate buffer / DMSO (4:1 v / v, pH 7.4 or 5.6), and set aside. The concentrations of Cys, Hcy and GSH solutions were prepared separately with deionized water to be 1×10 -2 M. With different concentrations of Cys, Hcy and GSH solutions added to the 5 μM fluorescent probe test solution, the excitation wavelengths were 350nm and 475nm respectively, and the fluorescence emission spectrum was tested. The measurement results are as follows: figure 2 shown.
[0048] from figure 2 It can be found from the results that when the pH is 7.4 and the excitation wavelength is 350nm, the fluorescence intensity at the emission wavelength of 508nm has a good linear relationship with Cys, Hcy and GSH in the concentration range of 0-60μM. The YF probe can detect the total concentra...
Embodiment 3
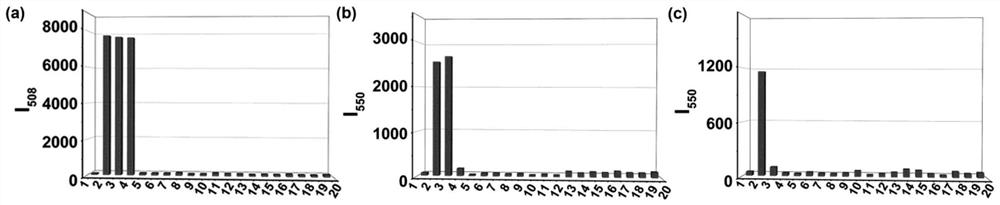
[0050] The selectivity of the fluorescent probes obtained in Example 1 to the fluorescence detection of Cys, Hcy and GSH.
[0051] Prepare test solution according to Example 2, prepare various analytes (Lys, Arg, Ser, Leu, Phe, Ala, Gly, Glu, Val, Gln, K with deionized water) + , Na + , Mg 2+ , Zn 2+ , H 2 o 2 ) The concentration of the solution is 1×10 -2 M. First add 12 equivalents of other possible interfering substances to the 5μM fluorescent probe molecular test solution, including blank, Lys, Arg, Ser, Leu, Phe, Ala, Gly, Glu, Val, Gln, K + , Na + , Mg 2+ , Zn 2+ , H 2 o 2 , and then add 12 equivalents of Cys, Hcy and GSH to these solutions respectively. After mixing for 10 minutes, under the same conditions, the excitation wavelengths of 350nm and 475nm were respectively used for fluorescence spectrum testing to obtain the fluorescence spectrum of each group of solutions.
[0052] from image 3 It can be found from the results that when the system is added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com