Multifunctional metalloporphyrin complex and preparation method thereof, and preparation method of polycarbonate
A metalloporphyrin and complex technology, applied in the preparation of polycarbonate, multifunctional metalloporphyrin complexes and the field of preparation thereof, can solve the problems of many cyclic by-products, difficult to control the composition ratio of polymer products, insufficient activity and the like , to achieve the effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] The present invention provides a kind of preparation method of polyfunctional metalloporphyrin complex described in above-mentioned technical scheme, comprises the following steps:
[0072] reacting the multifunctional ligand with the structure of formula III with the organic compound containing M and X groups to obtain the multifunctional metalloporphyrin complex with the structure of formula I;
[0073] The M is magnesium, aluminum, zinc, chromium, manganese, iron, cobalt, titanium, yttrium, nickel or ruthenium;
[0074] The X is halogen, -NO 3 、CH 3 COO-, CCl 3 COO-, CF 3 COO-, ClO 4 -, BF 4 -, BPh 4 -, -CN, -N 3 , p-toluate, p-toluenesulfonate, o-nitrophenol oxyanion, p-nitrophenol oxyanion, m-nitrophenol oxyanion, 2,4-dinitrophenol oxyanion, 3,5 Dinitrophenol oxyanion, 2,4,6-trinitrophenol oxyanion, 3,5-dichlorophenol oxyanion, 3,5-difluorophenol oxyanion, 3,5-di-trifluoromethyl Phenol oxyanion or pentafluorophenol oxyanion;
[0075]
[0076] R' is a s...
preparation example 1
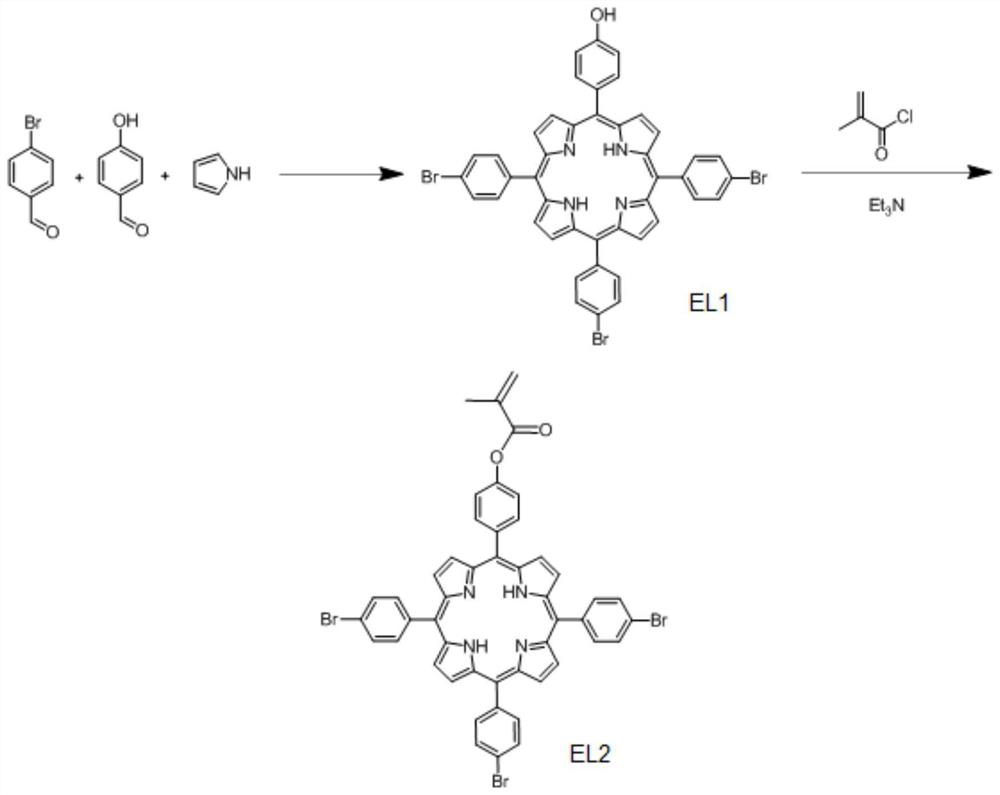
[0095] Add 15g (120mmol) of 3-hydroxybenzaldehyde, 68.1g (370mmol) of 4-bromobenzaldehyde and 33g (490mmol) of pyrrole into 500mL of propionic acid, heat up to about 130°C, reflux for 1.5h, and cool down after the reaction To room temperature, the reaction solution was concentrated to 200 mL, cooled overnight in the refrigerator after adding methanol, and the resulting product was filtered through silica gel column chromatography (CHCl 3 / CH 3 OH) to obtain the product EL1 with a yield of about 7.8%. 1 H-NMR (CDCl 3 , ppm): 8.9, 8.8, 8.1, 7.8, 7.2, -2.8. High-resolution electrospray mass spectrometry analysis, the analysis result is [C44H27Br3N4O]: 863.97, found: 863.86;
[0096] Under the protection of nitrogen, dissolve 2g (2.3mmol) of EL1 in 20mL of anhydrous THF, drop in 0.26g (2.5mmol) of methacryloyl chloride and 0.25g (2.5mmol) of triethylamine at low temperature, and mix thoroughly The above mixture was reacted at room temperature for 12h. After the reaction, the ...
preparation example 2
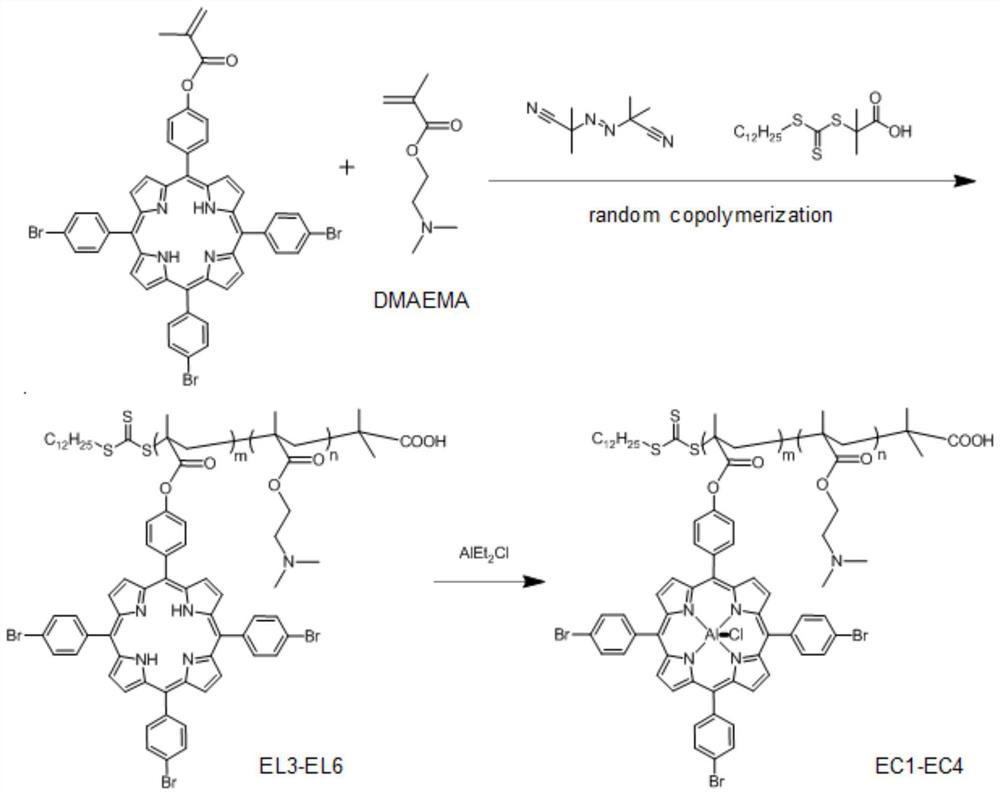
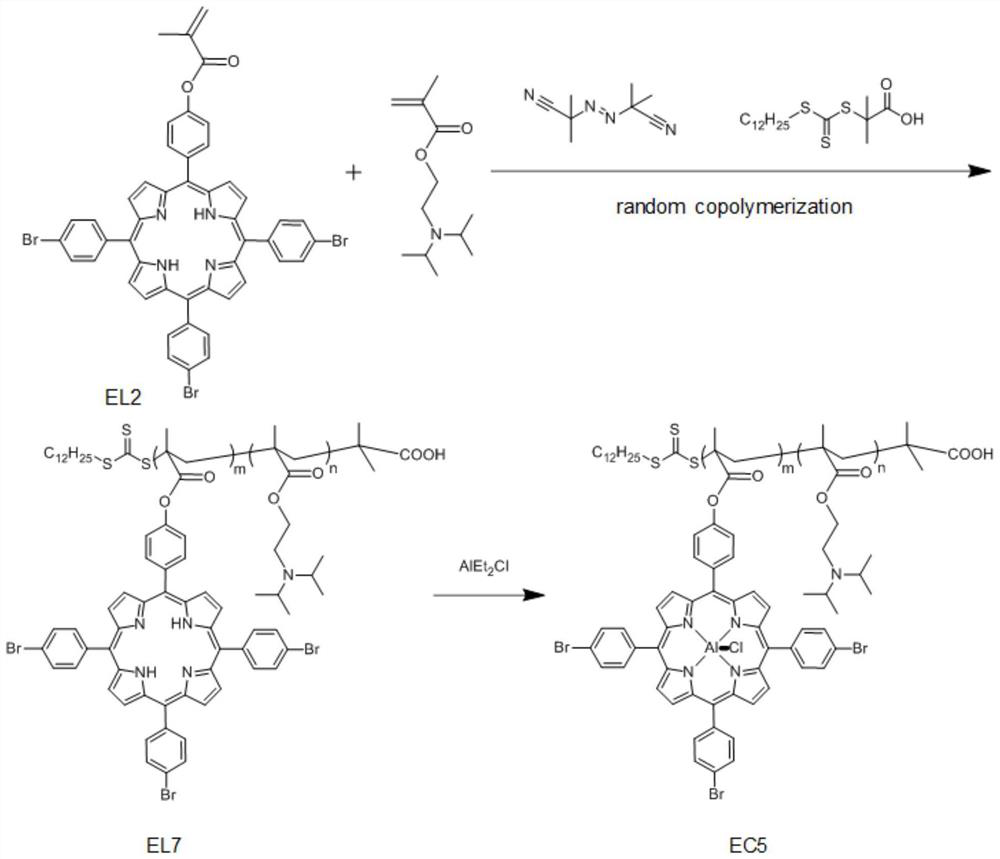
[0101]Under nitrogen protection, 1g (1.07mmol) EL2, 0.17g (1.07mmol) dimethylaminoethyl methacrylate (DMAEMA), 8.78mg (5.35×10 -2 mmol) azobisisobutyronitrile (AIBN) and 38.98mg (0.107mmol) RAFT reagent (DDMAP) were dissolved in 20mL of anhydrous THF, and after freezing-pumping-thawing three times to remove oxygen, the above mixture was heated to 70°C for 36h . After the reaction was completed, the reaction vessel was quenched and thawed in liquid nitrogen, and the precipitation was dissolved and precipitated repeatedly through the dichloromethane / ether system, and 0.72 g of multifunctional porphyrin ligand EL4 was obtained by isolation. 1 H NMR NMR and GPC tests show that m is 9 and n is 10;
[0102] Under the protection of nitrogen, the above ligand EL4 was dissolved in dichloromethane, and AlEt was added dropwise 2 Cl (diethylaluminum chloride), stirred at room temperature for 2 h. The obtained product was purified by column chromatography and dried to obtain the desired...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com