Porphyrin-selenide-adriamycin composite nanoparticles as well as preparation method and application thereof
A technology of composite nanoparticles and doxorubicin, which is applied in the direction of nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of failure to achieve therapeutic effect, liver function damage, and clinical application limitations, and achieve superior photothermal performance and reduce Toxic and side effects, the effect of achieving specific release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of Porphyrin-Selenide-Adriamycin Composite Nanoparticles
[0046] Step a) dissolving doxorubicin hydrochloride in water to obtain an aqueous solution of 1 mg / mL doxorubicin hydrochloride;
[0047] Step b) Weighing 5,10,15,20-tetrakis(4-aminobenzene)-21H,23H-porphyrin (0.338g, 0.5mmol), purchased from Jiangsu Aikang Biopharmaceutical Research and Development Co., Ltd., diselenide ( 0.368g, 1mmol), dissolved in 30mL of 1,4-dioxane solution, placed in a high-temperature glass tube, ultrasonically reacted for 30min, and air-sealed after three freezing pump-thawing cycles. After the reaction tube was reacted at a constant temperature of 120° C. for 24 hours, the hydrothermal reaction was completed. Cooled and opened, the purple product was isolated by filtration. During further purification, the purple unreacted product was eluted with tetrahydrofuran (THF), and the porphyrin-selenoether porous organic polymer was obtained after further vacuum drying;...
Embodiment 2
[0050] Example 2 Research on Morphological Characteristics and Characterization of Porphyrin-Selenide Porous Organic Polymer and Porphyrin-Selenide-Adriamycin Composite Nanoparticles
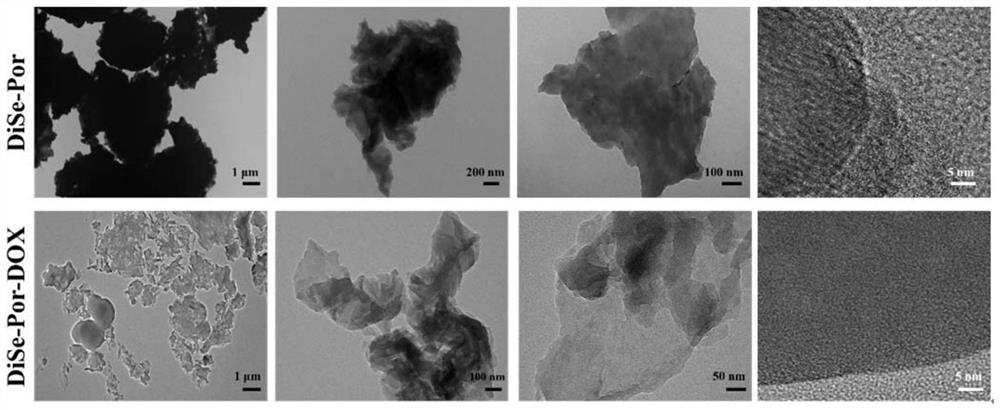
[0051] The porphyrin-selenoether porous organic polymer prepared in step b) of Example 1 and the porphyrin-selenoether-doxorubicin nanoparticles prepared in step d) of Example 1 were subjected to morphological research. The morphology was observed by transmission electron microscopy (TEM) at room temperature ( figure 1 ).
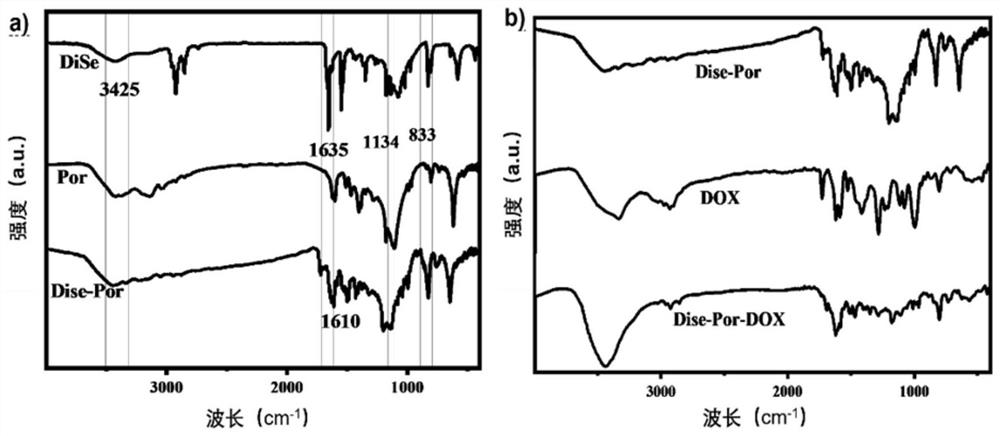
[0052] The infrared absorption spectrum of the samples was detected by the potassium bromide tablet method. Grind porphyrin-selenoether-doxorubicin polymer, doxorubicin, porphyrin-selenoether-doxorubicin nanoparticle solid powder with potassium bromide powder respectively, take 1-2mg sample and add 100-200mg bromine Potassium chloride is ground into a fine powder in an agate mortar, and the sample is continuously scraped to the center of the mortar with a small stainless st...
Embodiment 3 Embodiment 1
[0057] The porphyrin-selenoether-doxorubicin composite nanoparticle encapsulation efficiency, the mensuration of drug loading amount that embodiment 3 embodiment 1 makes
[0058] (1) Weigh an appropriate amount of doxorubicin reference substance, and after dissolving in distilled water, the ultraviolet spectrophotometer scans at 200-800 nm to obtain the maximum absorption wavelength at 483 nm.
[0059] (2) Drawing of standard curve
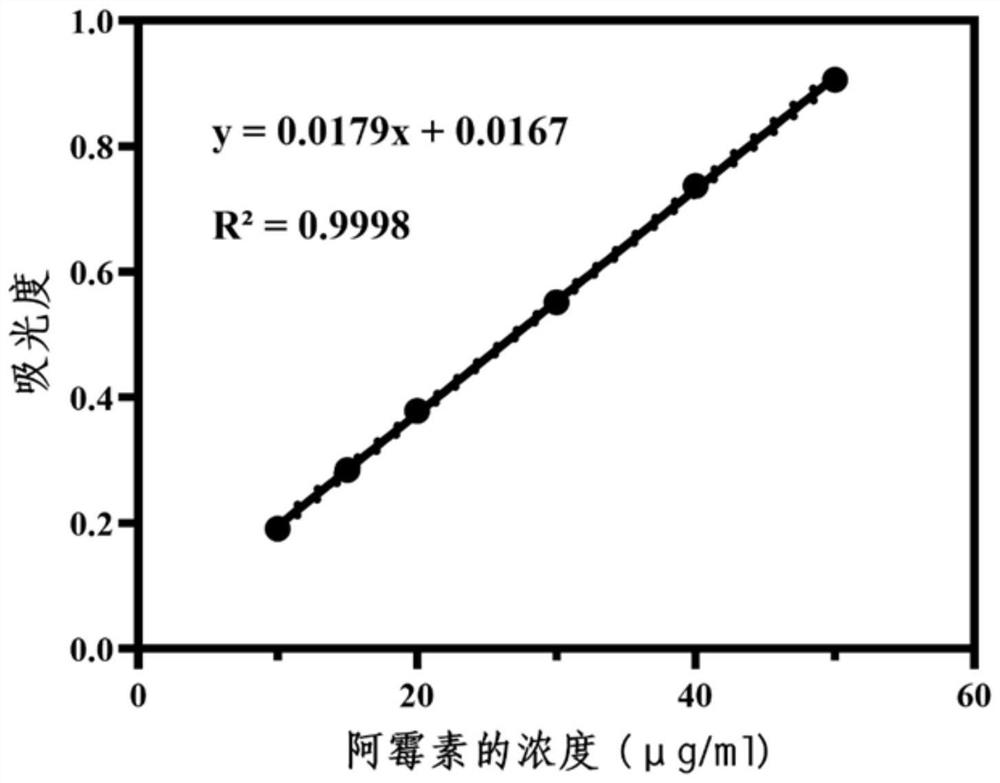
[0060] Prepare 0.5 g / L doxorubicin hydrochloride standard solution stock solution with distilled water. Obtain standard solutions with mass concentrations of 5.0, 10.0, 20.0, 30.0, 40.0, and 50.0 μg / L for the test solution. Measure the absorbance (A) value of different mass concentration doxorubicin hydrochloride test solution respectively at 483nm wavelength place, carry out linear regression to mass concentration with A value, regression equation is A=0.0179C+0.0167(R 2 =0.9998, n=3), the linear relationship is good.
[0061] (3) Determinatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com