High-sensitivity non-enzymatic glucose sensor and preparation method thereof
A glucose sensor and high-sensitivity technology, applied in the field of glucose sensors, can solve the problems of low working voltage biocompatibility and stability, poor biocompatibility of nano-copper oxide, low detection limit and working voltage, etc., and achieve good biological phase. Capacitive and corrosion resistance, large detection linear range, small detection limit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of Example 1 Gold@Bovine Serum Albumin
[0028] A. Slowly add 10ml of chloroauric acid solution with a concentration of 10mmol / L dropwise into 10ml of bovine serum albumin solution with a concentration of 5mg / mL in stirring, and stir for 5min.
[0029] B. Slowly add sodium hydroxide solution with a concentration of 0.2 mol / L dropwise, adjust the pH value to 12, and stir for 1 min.
[0030] C. Quickly add 50 mg of ascorbic acid and stir for 5 minutes.
[0031] D. Centrifuge the fully reacted solution at a speed of 8000r / min for 10min, wash the precipitate with deionized water and centrifuge, repeat the washing and centrifugation twice, and finally prepare 10ml of deionized water, disperse the centrifuged precipitate and seal it. As for aging at a low temperature of about 4°C for 24 hours, set aside for later use to obtain a gold@bovine serum albumin solution.
Embodiment 2
[0032] Embodiment 2 Preparation of high-sensitivity non-enzyme glucose sensor
[0033] A. Based on fluorine-doped tin oxide conductive glass, the specification is 25×8×1.1mm. Take 10 μL of the prepared gold@bovine serum albumin solution and drop it on the fluoride-doped tin oxide glass to form a circular drop with a diameter of about 5 mm.
[0034] B. Dry the fluorine-doped tin oxide conductive glass with droplets on it at 50°C until the droplets are completely dry to form a dark blue circular film.
[0035]C. Calcining the fluorine-doped tin oxide conductive glass dried in the previous step at a high temperature of 400°C for 2 hours, and after cooling to room temperature, the film takes on an obvious golden yellow color. So far, a high-sensitivity non-enzymatic glucose sensor of the present invention has been prepared.
Embodiment 3
[0036] Embodiment 3 detection experiment
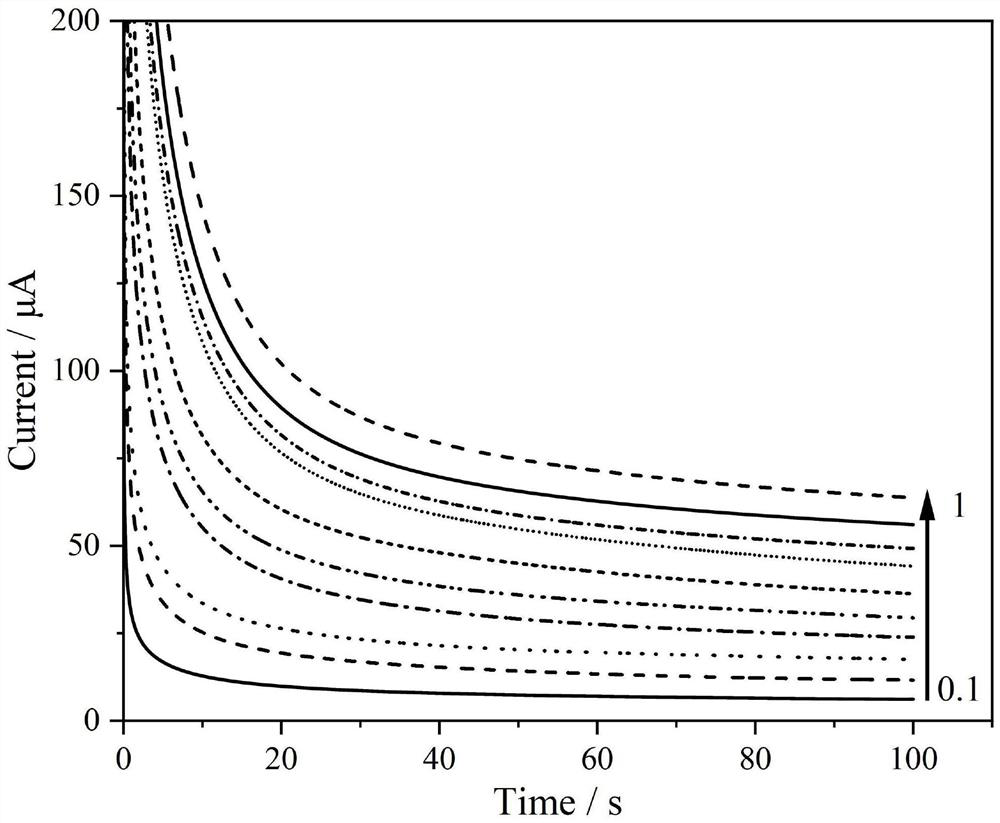
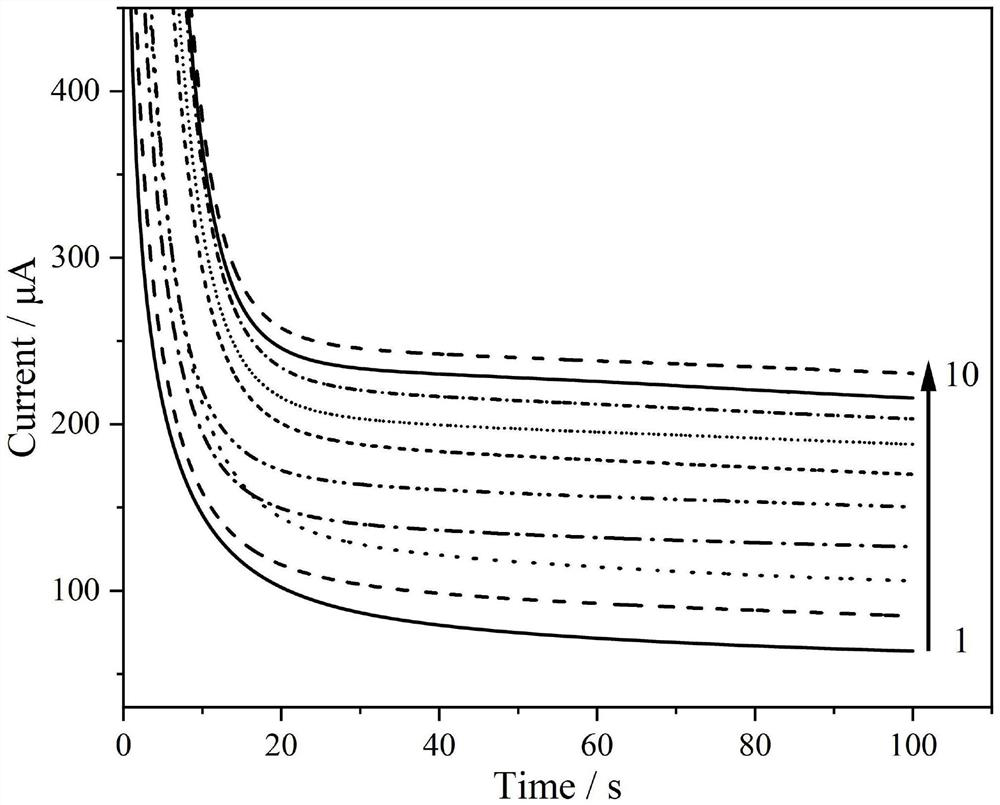
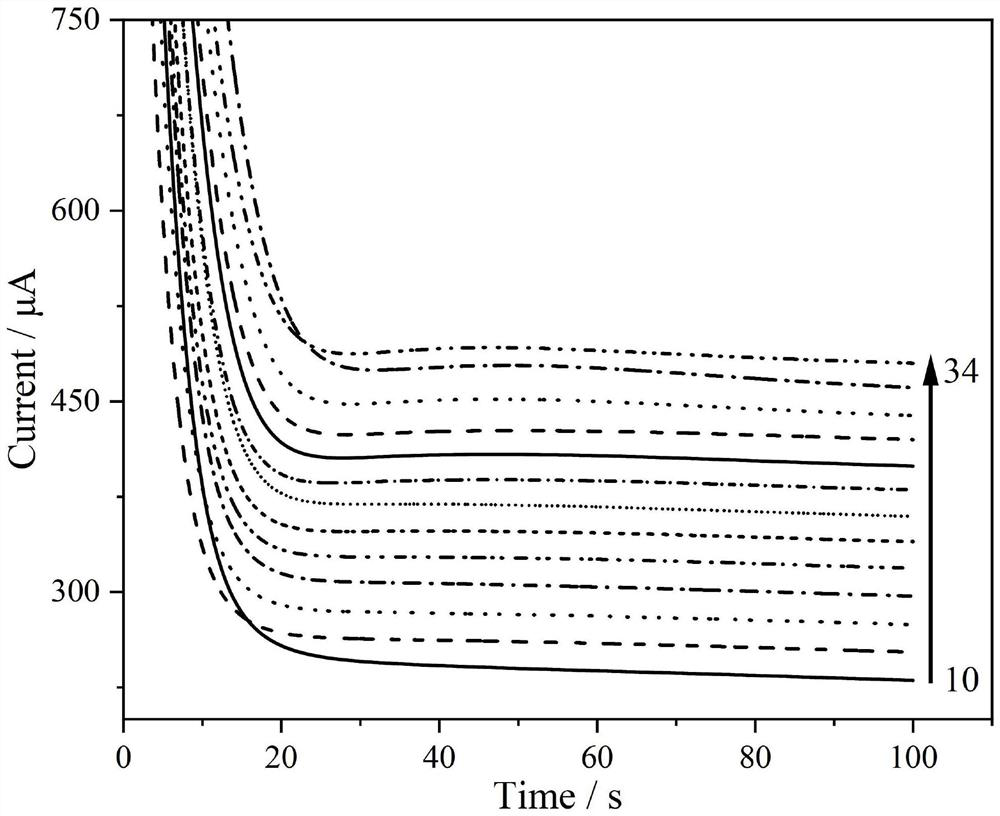
[0037] The detection experiment was carried out on a CHI600E electrochemical workstation and a three-electrode system. The working electrode is the high-sensitivity non-enzymatic glucose sensor of the present invention, the counter electrode is a platinum wire electrode, and the reference electrode is a saturated calomel electrode. First, activate the sensor in sulfuric acid with a concentration of 0.1mol / L, that is, scan the cyclic voltammetry curve. The start and end voltages are 0V and 1.5V respectively, and the cycle is more than 20 cycles, so that the adjacent two curves are almost overlapped, so far. activation. Then, use the single potential step chronoamperometry to characterize the sensor performance of the present invention, under the action potential of 0.15V, add glucose continuously in the bottom liquid of test, measure the current time curve under different concentrations, as figure 1 , figure 2 and image 3 shown. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com