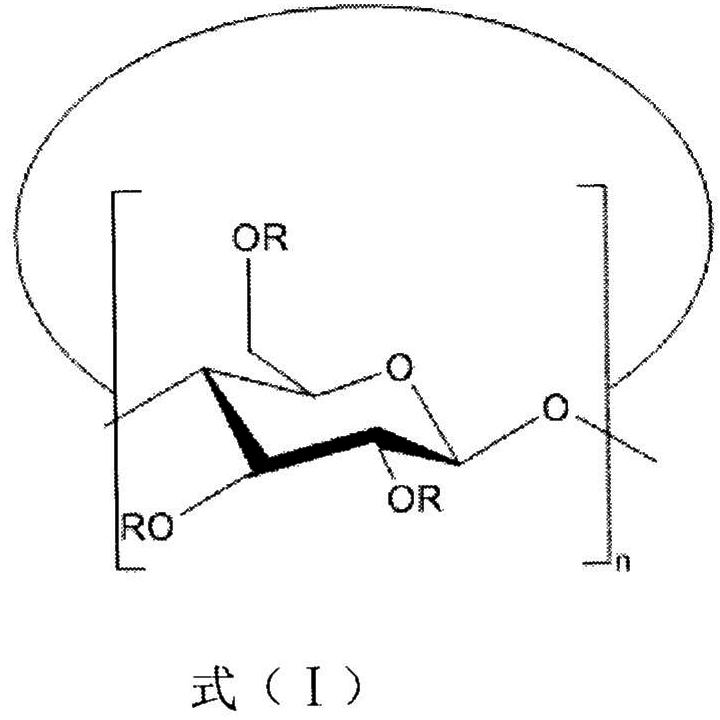
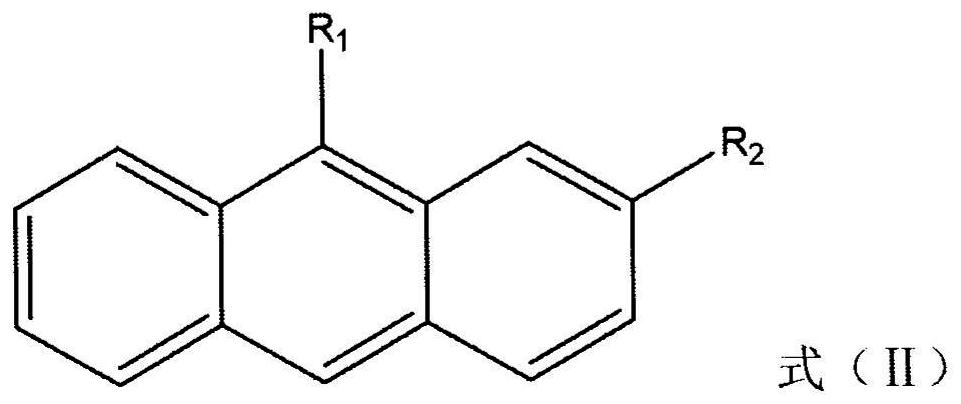
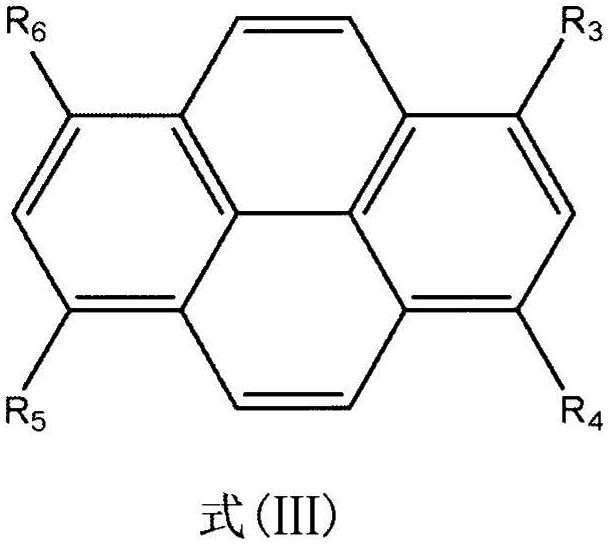
Cyclodextrin inclusion compound molecular glass photoresist
A cyclodextrin inclusion compound and molecular glass technology, applied in the field of photoresist, can solve the problem of resin solubility, irregular sawtooth, stuck neck, etc., to solve the stuck neck problem, improve the dry corrosion resistance, structure regular effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In a nitrogen-filled state, each glucose unit contains 1.6-2 methyl β-cyclodextrin (methyl β-cyclodextrin) 14.3 g (about 11 mmol), and 1-adamantanol 2 g (about 13 mmol) Mixed, heated to 250°C in a sealed state, shaken for 30 min and cooled to room temperature to obtain an inclusion resin containing 1-adamantanol by cyclodextrin; take 13.1 g (about 9 mmol) of the inclusion resin and 4-dimethylamino Pyridine 6.6g (about 54mmol) was dissolved in 300ml of anhydrous tetrahydrofuran, and then 18g (about 82mmol) of di-tert-butyl carbonic anhydride was added and stirred at room temperature for 20 hours. After 20 hours of stirring, an appropriate amount of deionized water was injected, the sediment was collected, and rinsed with sufficient deionized water. It was then dissolved in ethyl acetate, washed with saturated brine; dried over anhydrous magnesium sulfate, the solution was filtered and concentrated, and vacuum-dried at 50° C. for 12 hours to obtain molecular glass film-for...
Embodiment 2
[0044] Dissolve 13 g (about 10 mmol) of β-cyclodextrin (methyl β-cyclodextrin) containing 1.6 to 2 methyl groups in each glucose unit in 200 ml of deionized water, then add a saturated solution of o-carborane in tetrahydrofuran, and heat up. To 60 ℃ of stirring for 20 hours, the water is distilled off at a high temperature in a vacuum, and the obtained solid will be removed with chloroform to remove excess o-carborane to obtain a cyclodextrin inclusion o-carborane inclusion resin; get this inclusion resin 13g (about 9mmol) and 6.6g (about 54mmol) of 4-dimethylaminopyridine were dissolved in 300ml of anhydrous tetrahydrofuran, and then 18g (about 82mmol) of di-tert-butyl carbonate was added at room temperature and stirred for 20 hours. , rinsed with sufficient deionized water, dissolved in ethyl acetate, and washed with saturated brine; then dried over anhydrous magnesium sulfate, the solution was filtered and concentrated, and vacuum-dried at 50 °C for 12 hours to obtain a mole...
Embodiment 3
[0047] Dissolve 13 g (about 10 mmol) of β-cyclodextrin (methyl β-cyclodextrin) containing 1.6 to 2 methyl groups in each glucose unit into 200 ml of deionized water to form a solution, and then add 1,1 ferrocene formic acid 5.2 g (about 19 mmol) was dissolved in 100 ml of tetrahydrofuran, mixed into methyl β-cyclodextrin aqueous solution, heated to 50° C., stirred under 30KHz ultrasonic vibration for 20 hours, spray-dried, and the obtained solid was removed with chloroform to remove excess 1,1-dioxane ferrocene carboxylic acid to obtain an inclusion resin containing 1,1 ferrocene carboxylic acid with cyclodextrin; take 14.2 g (about 9 mmol) of the inclusion resin and 6.6 g (about 54 mmol) of 4-dimethylaminopyridine and dissolve it in 300 ml add 18 g (about 82 mmol) of di-tert-butyl carbonic anhydride, stir at room temperature for 20 hours, inject an appropriate amount of deionized water, collect the sediment, rinse with sufficient deionized water, dissolve in ethyl acetate, use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com