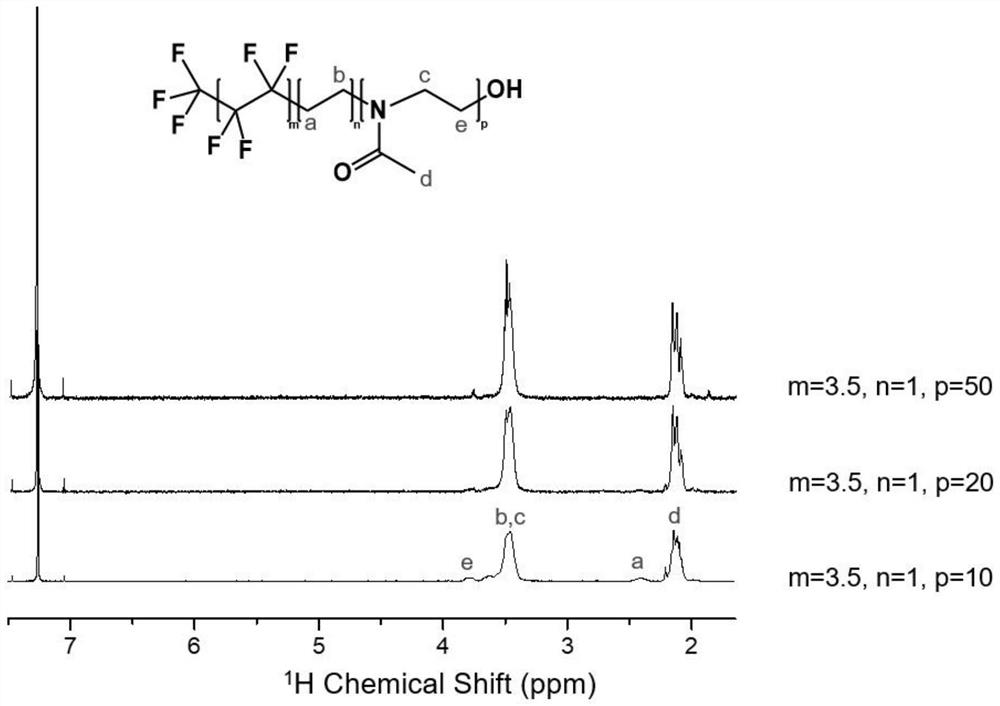
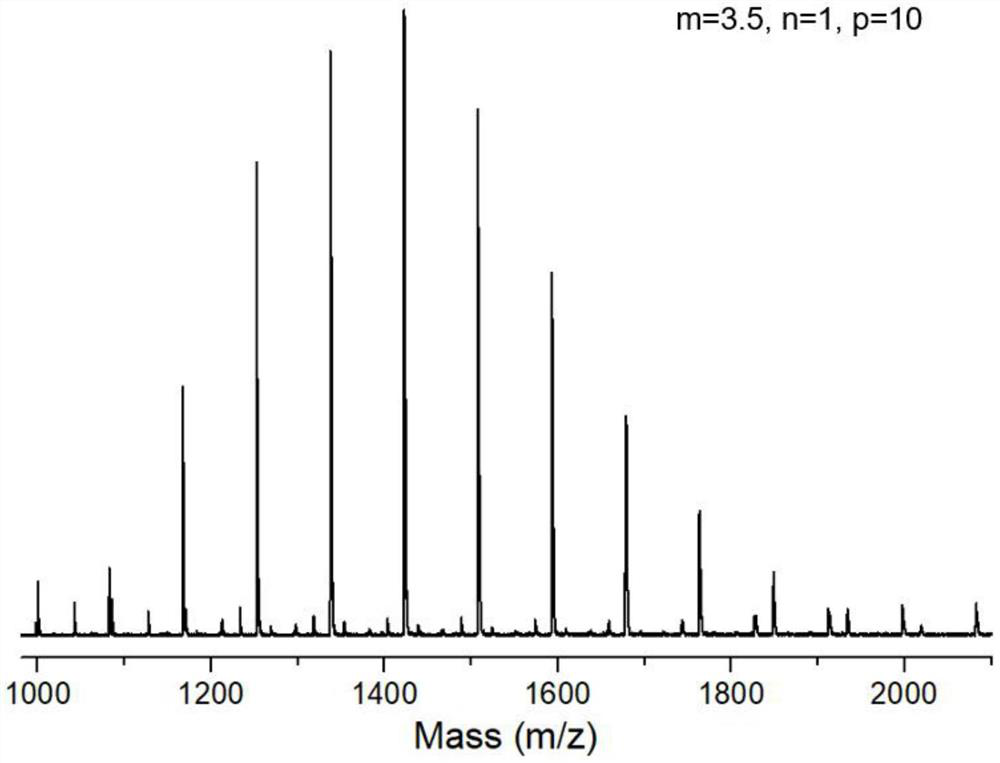
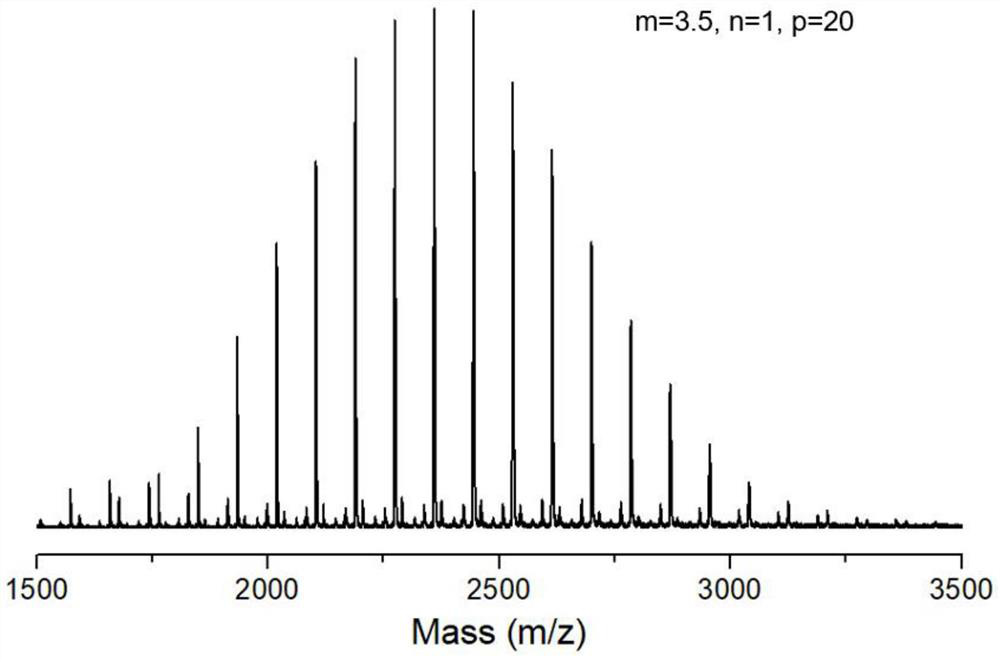
Fluorine-containing amphiphilic block copolymer, preparation and application thereof, fluorine-containing amphiphilic block copolymer hybrid proton exchange membrane and preparation thereof
A proton exchange membrane and amphiphilic block technology, which is applied in the field of fluorine-containing amphiphilic block copolymer hybrid proton exchange membrane and its preparation, can solve the problems of difficult dispersion, difficult synthesis, poor compatibility, etc., and achieve Good compatibility, effect of improving dimensional stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention provides the preparation method of the fluorine-containing amphiphilic block copolymer described in the above technical scheme, comprising the following steps:
[0034] Mix perfluoroalkyl alcohol, trifluoromethanesulfonic anhydride, auxiliary agent and first solvent, and carry out esterification reaction to obtain perfluoroalkyl trifluoromethanesulfonate; the structural formula of the perfluoroalkyl alcohol is F 3 C-(CF 2 -CF 2 ) m -(CH 2 -CH 2 ) n -OH; wherein, m=1~6, n=0.5~1;
[0035] The perfluoroalkyl trifluoromethanesulfonate, the oxazoline monomer and the second solvent are mixed, and ring-opening polymerization is carried out under anhydrous and oxygen-free conditions to obtain a fluorine-containing amphiphilic block copolymer; the oxazole The morpholine monomer is 2-methyl-2-oxazoline or 2-ethyl-2-oxazoline.
[0036] In the present invention, unless otherwise specified, the required preparation raw materials are all commercially availa...
Embodiment 1
[0077] Into a round-bottomed three-necked flask were added 1 g (2.16 mmol) of perfluoroalkyl alcohol, 0.36 mL (2.59 mmol) of dry triethylamine, 0.027 g (0.22 mmol) of 4-dimethylaminopyridine and 15 mL of dry dichloromethane , 0.435 mL (2.57 mmol) of trifluoromethanesulfonic anhydride was added dropwise under nitrogen protection, under the condition of ice-salt bath at -10 °C, and the dropwise addition process continued for 0.5 h; after the dropwise addition, the reaction was continued in the ice-salt bath environment for 40 min, and then removed Ice-salt bath, continue to react at 25°C for 12h;
[0078] After the reaction was completed, 10 mL of ice water was added to the reaction system to quench, and the liquids were separated, and the organic phase was collected and subjected to rotary evaporation under vacuum conditions. , to obtain the ether solution of the crude product; 20 mL of the ether solution of the crude product was washed with 10 mL of ice water each time, repeat...
Embodiment 2
[0084] Prepare perfluoroalkyl trifluoromethanesulfonate according to the method of embodiment 1;
[0085] Combine 0.3 g (0.50 mmol) of perfluoroalkyl trifluoromethanesulfonate with 0.865 mL (10 mmol) of 2-methyl-2-oxazoline monomer (oxazoline monomer and perfluoroalkyl trifluoromethanesulfonate) The molar ratio of oxazoline is 20:1), mixed with 1.65 mL of absolutely dry acetonitrile, so that the concentration of oxazoline monomer is 4 mol / L, under the protection of anhydrous and oxygen-free argon, after cationic ring-opening polymerization reaction is carried out in an oil bath at 80 °C for 10 h, the The reaction system was cooled to room temperature, and 7.5 mL of a methanol solution of 0.1 mol / L potassium hydroxide was added with a syringe. The molar ratio of potassium hydroxide to perfluoroalkyl trifluoromethanesulfonate was 1.5:1, under argon protection and room temperature conditions. to terminate for 4h;
[0086] After the termination, rotary evaporation was performed u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com