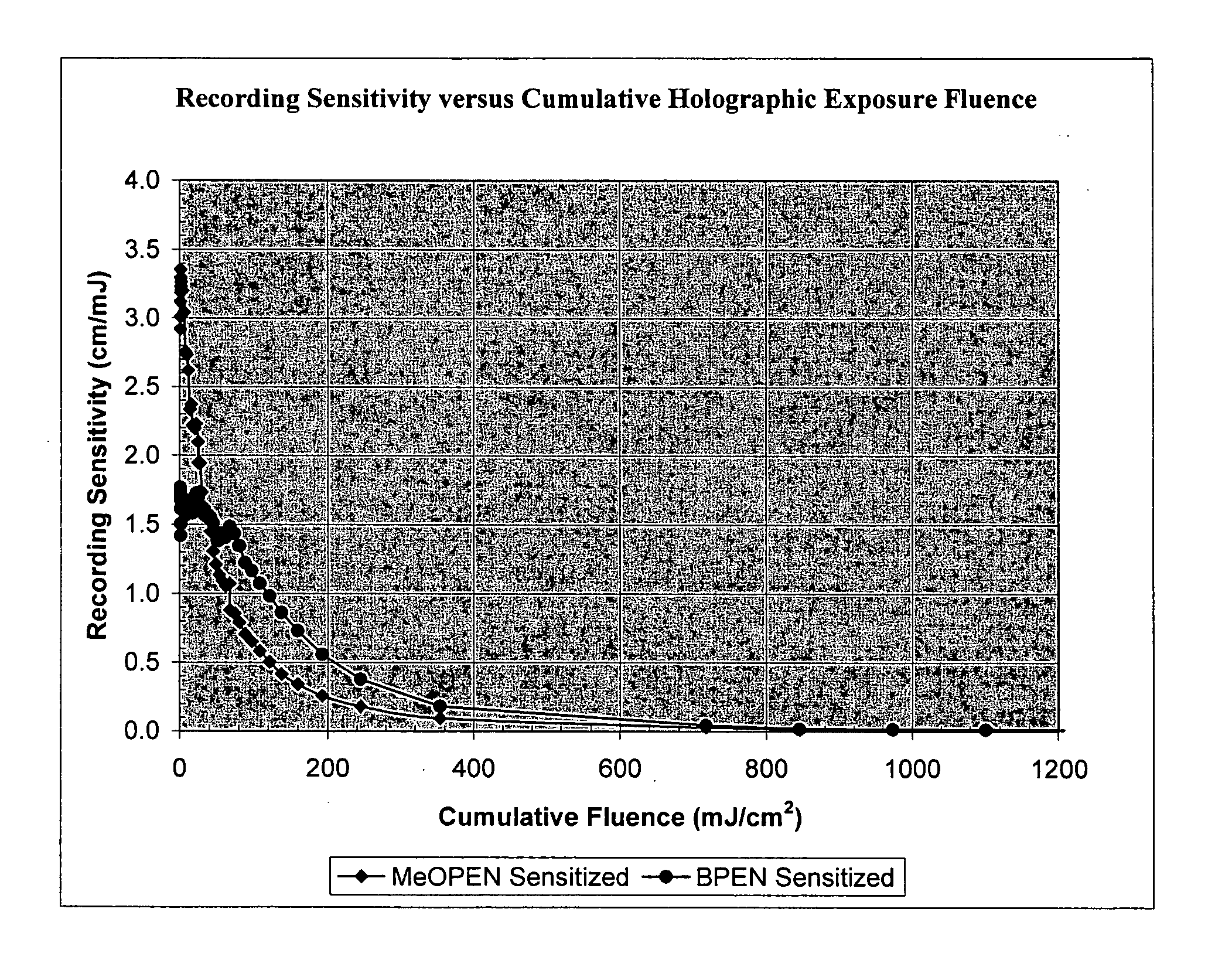
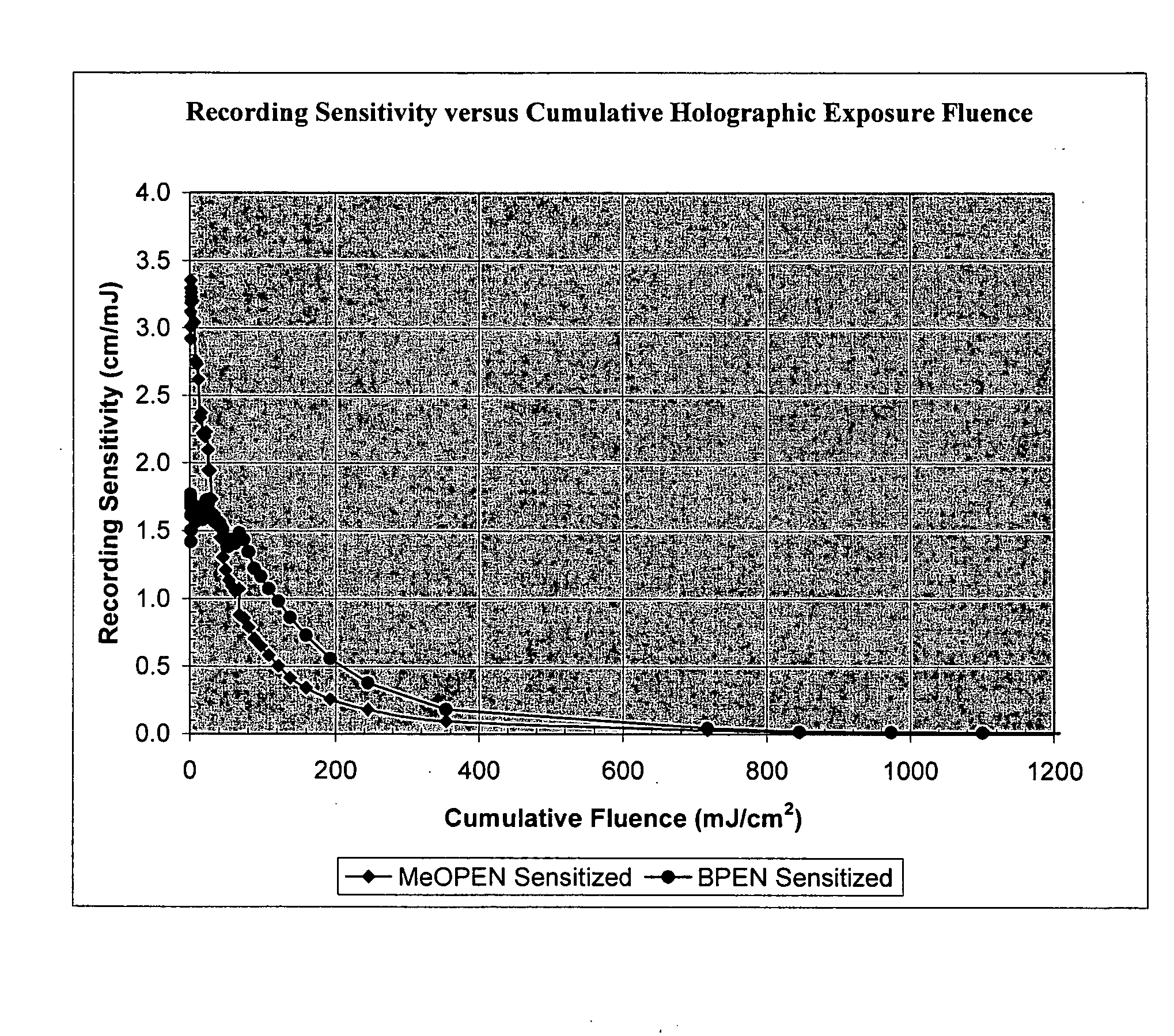
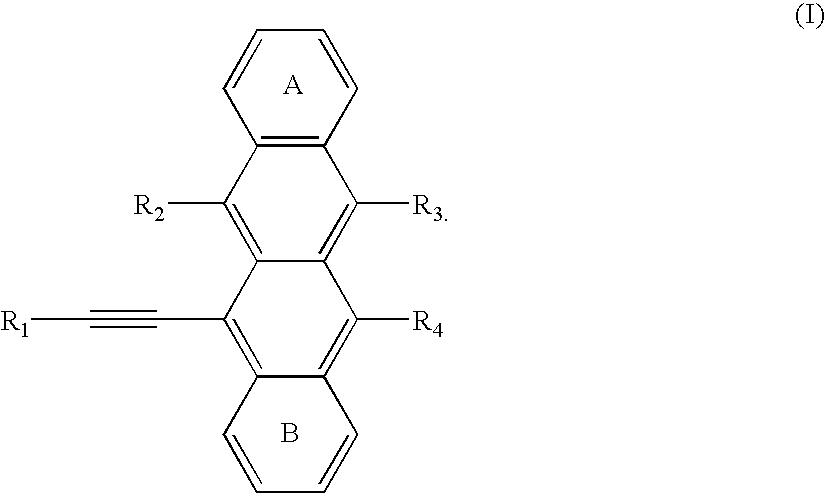
Sensitizer dyes for photoacid generating systems
a technology of sensitizer dyes and photoacids, applied in the field of photoresists and cationic polymerization, can solve the problems of increasing the optical path length of the medium, increasing the absorption of medium, etc., and achieves low extinction coefficient, good recording sensitivity, and efficient photosensitizers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0075] Preparation of A Disubstitued Naphthacene Dye
Step 1: 5-Bromonaphthacene
[0076] This intermediate dye was prepared by modifying the method described in J. Org. Chem., 1970, 35 (5), 1315-18. To an oven-dried 200-ml single-neck round-bottomed flask equipped with a magnetic stirrer and purged with nitrogen were added 1.16 g of naphthacene (Aldrich, 2,3-benzanthracene, C18H12, 5.1 mmol), followed by 100 ml carbon tetrachloride and 1.6 g copper(I) bromide (Aldrich, CuBr2, 7.2 mmol) to form a suspension. A reflux condenser was added to the flask and the mixture was refluxed at 85° C. for 24 hours.
[0077] After the reaction was cooled to room temperature, the solution was filtered and the solids were rinsed with additional CCl4. The solution was concentrated by rotary evaporation to about 50 ml, and passed through a column of neutral alumina (2×12 cm). The solvent was completely evaporated off and the product was dried under vacuum to yield 1.60 g (61%).
[0078] UV-Vis analysis of t...
example 2
[0087] Preparation of Additional Disubstituted Naphthacene Dyes
[0088] Similar dyes were synthesized in exactly the same manner as in Example 1. In step 4, phenylacetylene was substituted with trimethylsilylacetylene (R═(CH3)3 Si), 1-ethynyl-cyclohexene (R═C6H9), or t-butylacetylene (R═C4H9).
[0089] UV-Vis analysis of the product where R is C6H9 using HPLC with the product dissolved in 5% dichloromethane / hexanes showed a λmax at 451 nm, 481 nm and 515 nm.
[0090] UV-Vis analysis of the product where R is C4H9 using HPLC with the product dissolved in 5% dichloromethane / hexanes showed a λmax at 444 nm, 472 nm and 505 nm.
example 3
[0091] Synthesis of A Monosubstituted Naphthacene Dye
Synthesis of 5-(phenylethynyl)naphthacene
[0092] 5-Bromonaphthacene (1.00 g, 3.26 mmol, prepared as described in Example 1, Step 1), 31 mg of copper(I) iodide (0.163 mmol), 85 mg of triphenylphosphine (0.33 mmol) and 114 mg of dichlorobis(triphenylphosphine)palladium(II) catalyst (0.163 mmol) were added to an oven-dried glass pressure tube equipped with a magnetic stirrer. Dry triethylamine (20 ml) was added and the mixture was degassed with nitrogen for 15 minutes. Phenylacetylene (0.5 g, 0.46 ml, 4.88 mmol) was then added by syringe. The tube was sealed and heated in an oil bath to 95° C. while covered with aluminum foil to exclude light (for the duration of the reaction and work up, exposure to light was minimized). The reaction mixture was heated for two hours, and then allowed to cool slowly. The tube contents were dark red with a white precipitate. Upon further cooling, a dark red precipitate formed. TLC analysis with 30% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com