Molecular semiconductors containing diketopyrrolopyrrole and dithioketopyrrolopyrrole chromophores for small molecule or vapor processed solar cells
a technology of pyrrolopyrrole and chromophores, which is applied in the direction of semiconductor devices, electrical devices, nanotechnology, etc., can solve the problems of difficult to synthesize “good” p3ht, large amount of sunlight wasted in this system, and high molecular weight and high regioregularity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compound 4
[0167]Compound 4 was synthesized according to the following synthetic procedure:
[0168]Part A. The parent 2,5-dihydro-1,4-dioxo-3,6-dithienylpyrrolo[3,4-c]-pyrrole (1) was prepared in 55% yield following a previously reported procedure which comprises the reaction of 2-thiophenecarbonitrile with 0.5 eq of di-n-butyl succinate ester and an excess of potassium t-butoxide using 2-methyl-2-butanol as solvent. See Tamayo et al., J. Phys. Chem. C 112:15543 (2008) and references therein.
[0169]Part B. Compound (2) was synthesized according to a modified literature procedure. Zambounis et al. Nature 1997, 388, 131. In a three-necked, oven-dried 100 mL round bottom flask, 1 (3.0 g, 10.0 mmol) was dissolved in 150 mL of anhydrous tetrahydrofuran (THF) and the resulting solution was purged with argon for ten minutes. Dimethylaminopyridine (DMAP 3.0 g, 25 mmol) was added and the reaction mixture was stirred for 15 minutes under argon at room temperature. Di-tert-butyl-dicar...
example 2
Device Fabrication
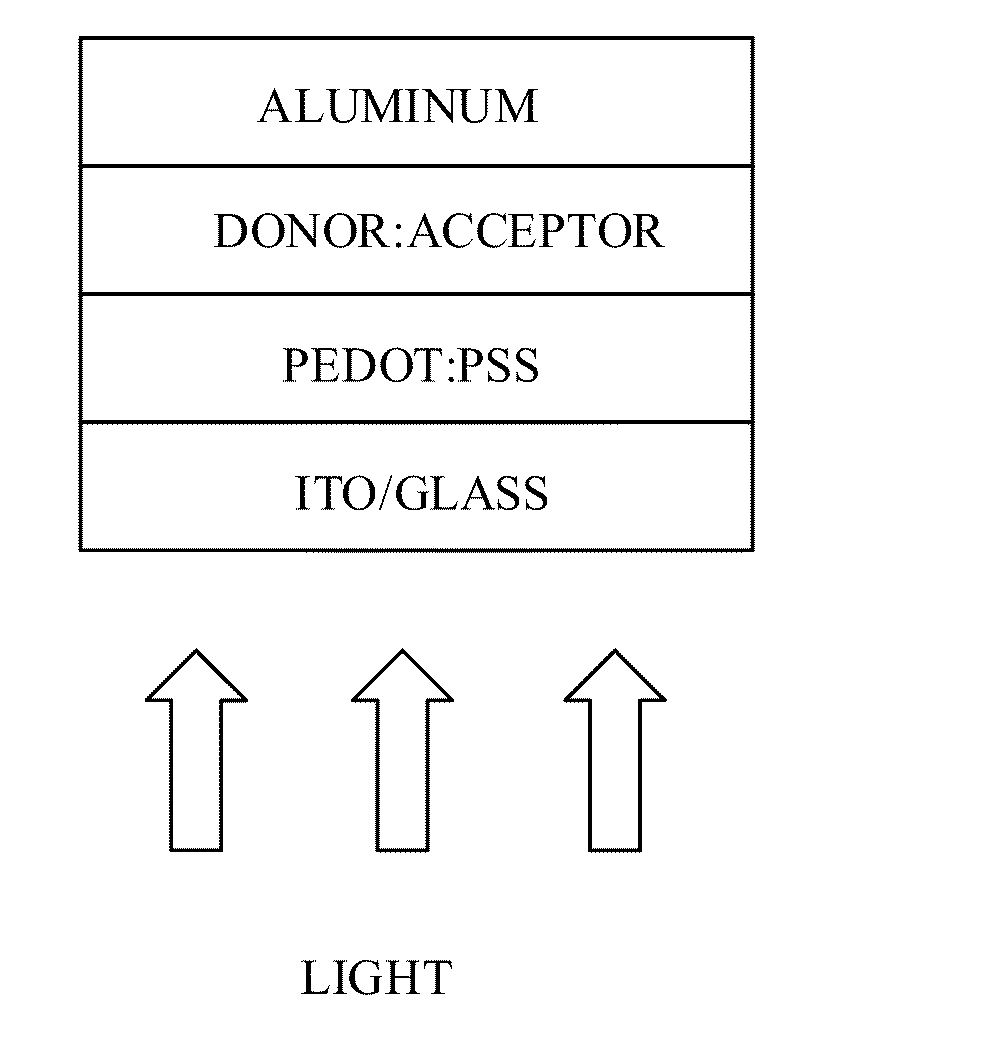
[0175]Indium tin oxide (ITO)-coated glass substrates (Thin Film Devices) were cleaned with detergent and de-ionized water after which the substrates were sonicated for 10 minutes in soap solution, de-ionized water, acetone and isopropanol. The ITO substrates were then treated in a UV ozone cleaner for 30 minutes followed by spin coating a solution of poly(3,4-ethylene dioxythiophene:poly(styrenesulfonate) (PEDOT:PSS, Baytron P) (5000 rpm for 40 seconds). The PEDOT:PSS film was dried at 140° C. inside a glovebox for 15 minutes which yielded a film 60 nm thick. A 2% (w / v) blend solution of compound (4) and PCBM (Nano-C, USA) in chloroform (CHCl3) was filtered through a 0.45 μm poly(tetrafluoroethylene) (PTFE) filter and spin coated at 1500 rpm for 60 seconds on top of the PEDOT:PSS layer. Subsequently, aluminum (1200 Å) was thermally evaporated at a pressure of 1×10−7 Torr at room temperature using a shadow mask. Illumination was done through the glass slide using li...
example 3
Electrochemical and Photophysical Characterization of (4)
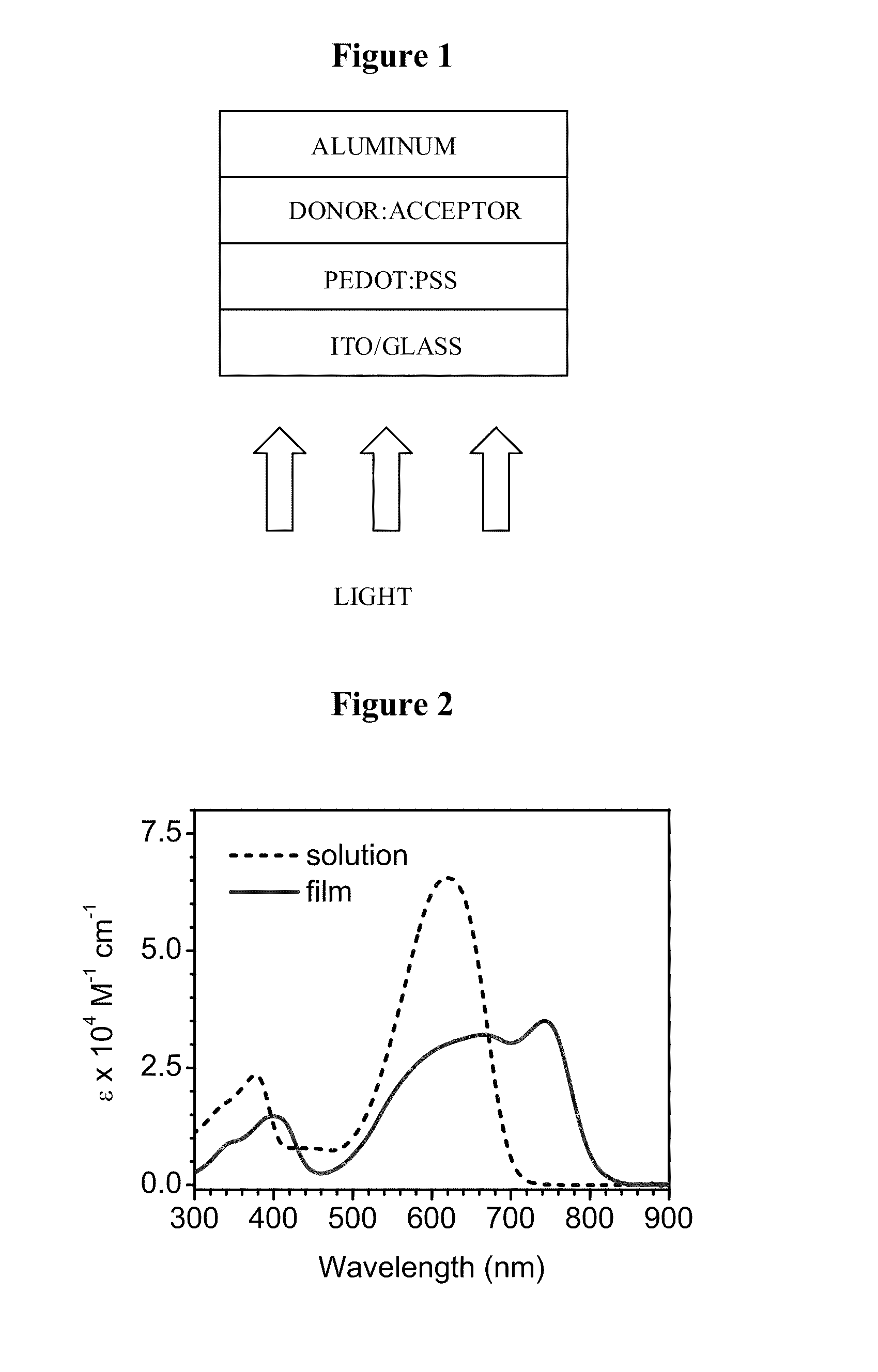
[0176]Cyclic voltammetry (CV) was performed using an EG&G potentiostat / galvanostat model 283. Anhydrous dichloromethane was used as the solvent under an inert atmosphere, and 0.1 M solution tetra-butyl ammonium hexafluorophosphate was used as the supporting electrolyte. A glassy carbon rod was used as the working electrode, a platinum wire was used as the counter electrode, and a silver wire was used as a pseudo reference electrode. The redox potentials are obtained by taking the average of anodic and cathodic waves and are reported relative to a ferrocenium / ferrocene (Cp2Fe+ / Cp2Fe, 0.475 V versus SCE in dichloromethane) redox couple used as an internal reference. UV-visible absorption spectra were recorded on a Shimadzu UV-2401 PC dual beam spectrometer. Steady-state fluorescence experiments at room temperature were performed using a PT1 (Lawrenceville, N.J.) Quantum Master fluorimeter equipped with a Xenon lamp excitation so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com