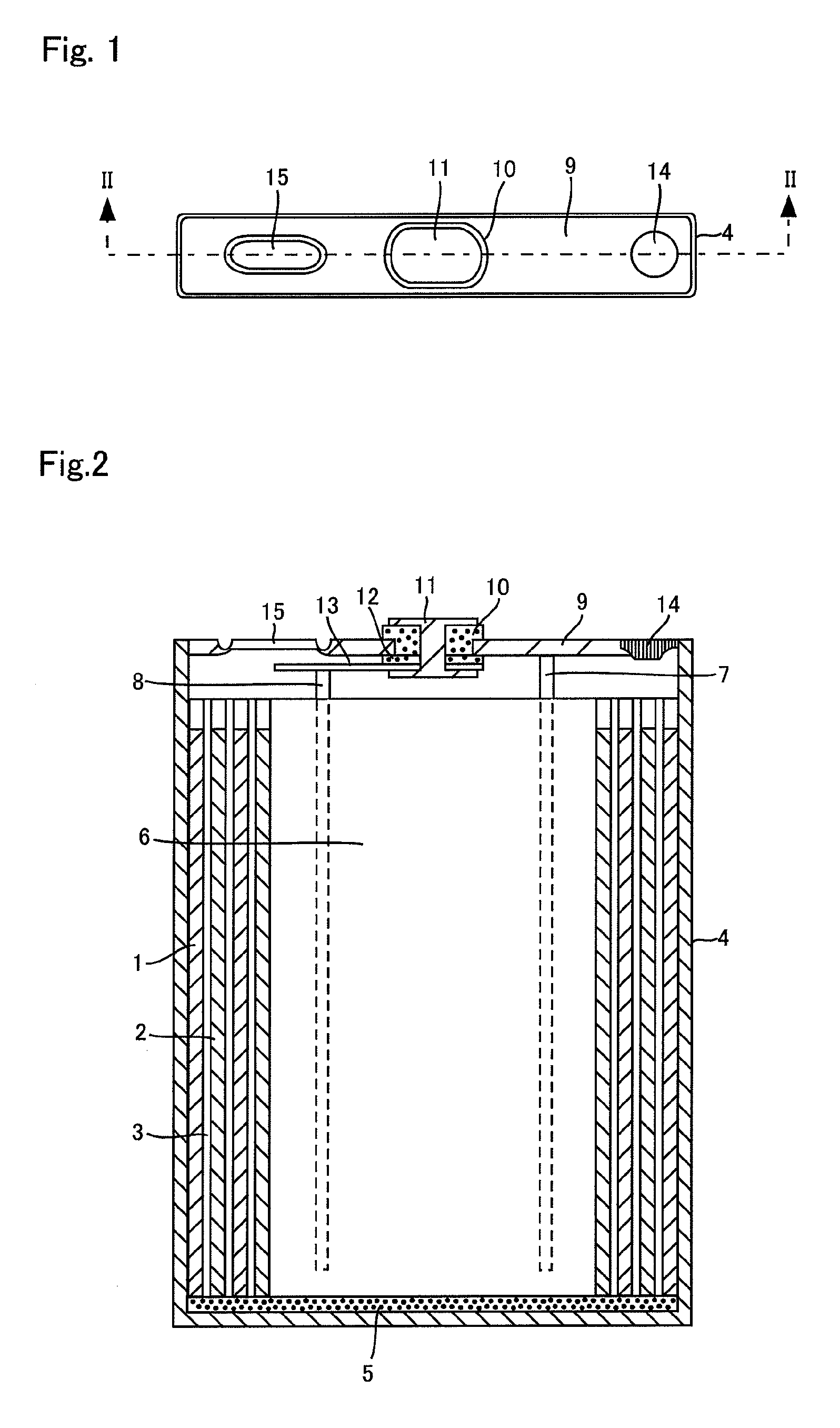
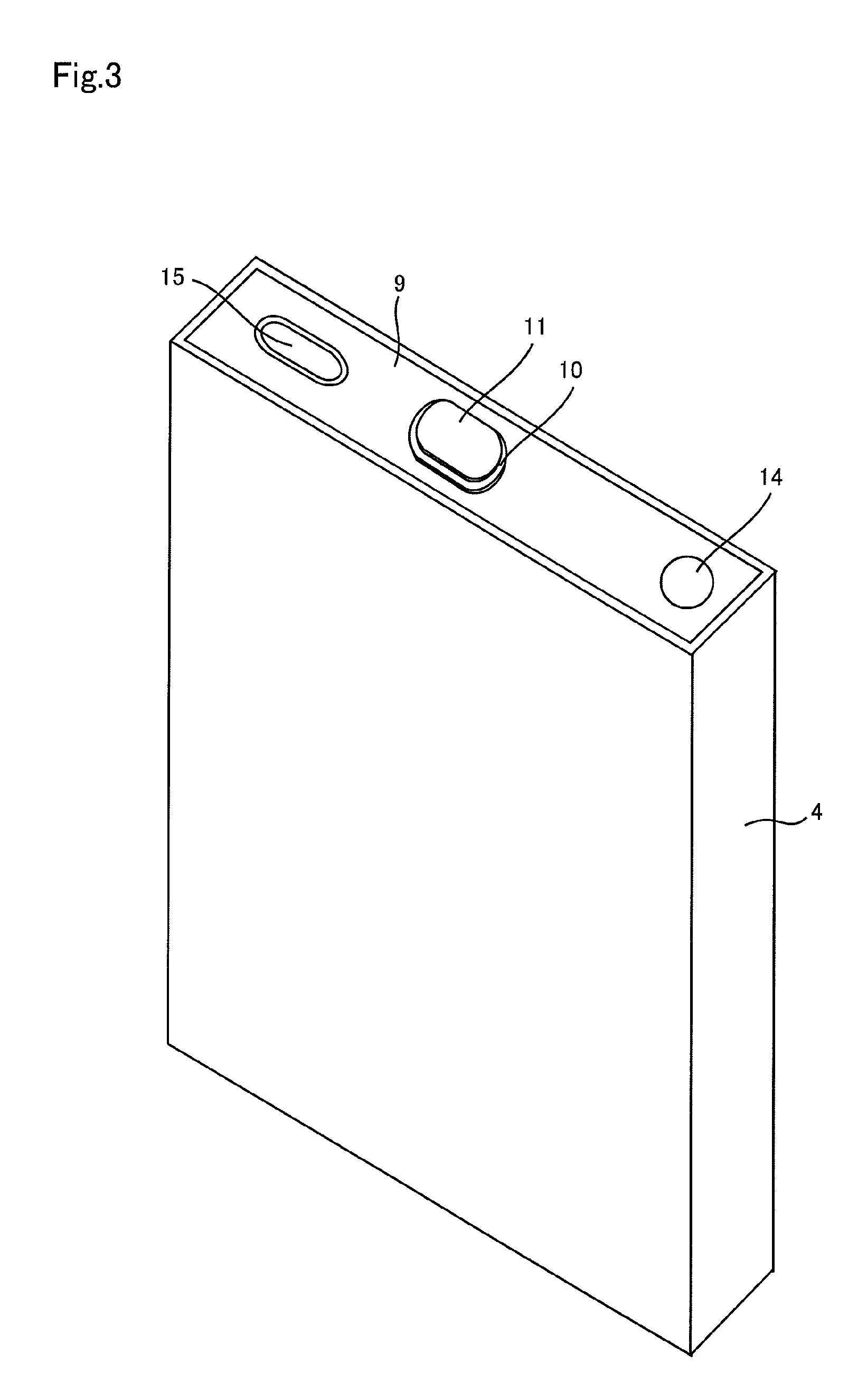
Positive electrode material, manufacturing method thereof, positive electrode for non-aqueous rechargeable battery, and non-aqueous rechargeable battery
a technology of positive electrode active material and manufacturing method, which is applied in the direction of cell components, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of lithium nickelate not providing satisfactory properties, lithium nickelate has a less stable crystal structure, and cannot be used to make sufficiently safe batteries, etc., to achieve the effect of reducing the amount of moisture adhered to the active material of the positive electrode in the positive electrode mixture containing composition, reducing the amount of moisture adhered to the active material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
[0025]A positive electrode material according to a first embodiment of the present invention includes at least a lithium nickel composite oxide represented by the following general compositional formula (1) as a positive electrode active material.
Li1+xMO2 (1)
where −0.5≦x≦0.5 and M represents a group of at least two elements including at least one of Mn and Co and Ni, wherein 20≦a≦100 and 50≦a+b+c≦100 for a, b and c being the ratios (mol %) of Ni, Mn, and Co, respectively among the elements that constitute M.
[0026]The lithium nickel composite oxide represented by the general compositional formula (1) includes an element group M including at least one selected from Mn and Co and Ni. Among the elements, Ni is a component that contributes to improvement in the capacity of the lithium nickel composite oxide.
[0027]It is preferable that Ni has a large ratio in the general compositional formula (1) that represents the lithium nickel composite oxide in order to achieve high capacity. Theref...
##ventive example 1
Inventive Example 1
Synthesizing Positive Electrode Active Material
[0095]Ammonia water having its pH adjusted to about 12 by addition of sodium hydroxide was placed into a reaction container, strongly stirred while a mixture aqueous solution containing nickel sulfate, manganese sulfate, and cobalt sulfate in concentrations of 2.4 mol / dm3, 0.8 mol / dm3, and 0.8 mol / dm3, respectively and ammonia water in a concentration of 25% by mass were dropped using a constant rate pump in ratios of 23 cm3 / min and 6.6 cm3 / min, respectively to synthesize a coprecipitation compound (spherical coprecipitation compound) of Ni, Mn, and Co. Note that at the time, the temperature of the reaction liquid was kept at 50° C., and a sodium hydroxide aqueous solution in a concentration of 6.4 mol / dm3 was concurrently dropped and a nitrogen gas was bubbled at a flow rate of 1 dm3 / min so that the pH of the reaction liquid was kept close to 12.
[0096]The coprecipitation compound was washed with water, filtered and d...
##ventive example 2
Inventive Example 2
[0116]Ammonia water having its pH adjusted to about 12 by adding sodium hydroxide was placed in a reaction container and strongly stirred, into which a mixture aqueous solution including nickel sulfate, cobalt sulfate, and aluminum sulfate in concentrations of 3.28 mol / dm3, 0.6 mol / dm3, and 0.12 mol / dm3, respectively, and 25% by mass of ammonia water were dropped in ratios of 23 cm3 / min and 6.6 cm3 / min, respectively using a constant rate pump, and a coprecipitation compound (spherical coprecipitation) of Ni, Co, and Al was synthesized. At the time, a sodium hydroxide aqueous solution in a concentration of 6.4 mol / dm3 was concurrently dropped and nitrogen gas was bubbled at a flow rate of 1 dm3 / min so that the pH of the reaction liquid was kept close to 12.
[0117]The coprecipitation compound was washed with water, filtered and dried, and a hydroxide containing Ni, Co, and Al in a mol ratio of 82:15:3 was obtained. 0.196 mol of the hydroxide and 0.204 mol of LiOH.H2O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com